Answer:
Butan-1-ol
contains an O-H group and hence undergoes intermolecular H-bonding.
 Therefore, its b.p. is much higher than that of butanal
Therefore, its b.p. is much higher than that of butanal ![]() which does not contain
an O-H group and hence does not undergo H-bonding. Instead it has only weak
dipole-dipole interactions.
which does not contain
an O-H group and hence does not undergo H-bonding. Instead it has only weak
dipole-dipole interactions.
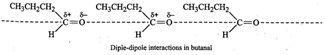
You need to login to perform this action.
You will be redirected in
3 sec
