Answer:
Since
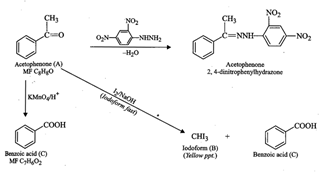
aromatic compound 'A' (MF ![]() )
gives positive 2, 4-DNP test, it must be an aldehyde or a ketone.
(ii) Since compound 'A' does not give Tollens' test or Fehling?s test,
therefore, 'A' must be a ketone.
(iii) Since compound 'A' on treatment with
)
gives positive 2, 4-DNP test, it must be an aldehyde or a ketone.
(ii) Since compound 'A' does not give Tollens' test or Fehling?s test,
therefore, 'A' must be a ketone.
(iii) Since compound 'A' on treatment with ![]() , gives yellow ppt. of
compound 'B', therefore, compound ?B? must be iodoform and the ketone 'A' must
be a methyl ketone.
(iv) Since methyl ketone 'A' on drastic oxidation with
KMn04 gives a carboxylic acid 'C'
, gives yellow ppt. of
compound 'B', therefore, compound ?B? must be iodoform and the ketone 'A' must
be a methyl ketone.
(iv) Since methyl ketone 'A' on drastic oxidation with
KMn04 gives a carboxylic acid 'C'![]() therefore, 'C' must be
benzole acid and compound 'A' must be acetophenone
therefore, 'C' must be
benzole acid and compound 'A' must be acetophenone ![]() (v) If 'A' is acetophenone, then all the reactions
described above may be explained as follows
(v) If 'A' is acetophenone, then all the reactions
described above may be explained as follows
You need to login to perform this action.
You will be redirected in
3 sec
