Answer:
(i) In aniline, the electron pair on nitrogen atom is
involvedin conjugation with ring and is less available for protonation than in
methyl amine. Therefore, ![]() value of aniline is more
than thatof methylamine and aniline is less basic. (As higher the
value of aniline is more
than thatof methylamine and aniline is less basic. (As higher the ![]() value,less
is the basicity).
(ii) Ethylamine is soluble in water due
to hydrogen bonding.
value,less
is the basicity).
(ii) Ethylamine is soluble in water due
to hydrogen bonding.
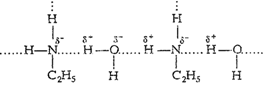 In aniline due to bulky hydrocarbon
part, the extent of hydrogenbonding is less and it is not soluble in water.
(iii) Methylamine being more
basic than water, accepts a proton from water and
In aniline due to bulky hydrocarbon
part, the extent of hydrogenbonding is less and it is not soluble in water.
(iii) Methylamine being more
basic than water, accepts a proton from water and ![]() ions are produced.
ions are produced.
![]()
![]()
![]() (iv) In presence of cone.
(iv) In presence of cone. ![]() and
cone.
and
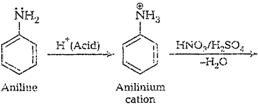
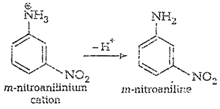
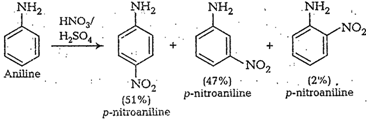
cone. ![]() most of aniline
getsprotonated to form anilinium ion. Now aniline and anilimum ionsboth are
present in the reaction mixture. In aniline
most of aniline
getsprotonated to form anilinium ion. Now aniline and anilimum ionsboth are
present in the reaction mixture. In aniline![]() group iso- and
p-directing but anilinium ion is m-directing. Because ormore amount of
anilinium ion, a lot of m-nitroaniline is formed.
group iso- and
p-directing but anilinium ion is m-directing. Because ormore amount of
anilinium ion, a lot of m-nitroaniline is formed.
 (v) Aniline being a Lewis base forms a
complex with
(v) Aniline being a Lewis base forms a
complex with ![]() a Lewisacid. As a
result, nitrogen of aniline becomes positively chargedand acts as a strong
deactivating group for electrophilicsubstitution reaction. Consequently,
aniline does not undergoFriedel Crafts reaction.
a Lewisacid. As a
result, nitrogen of aniline becomes positively chargedand acts as a strong
deactivating group for electrophilicsubstitution reaction. Consequently,
aniline does not undergoFriedel Crafts reaction.
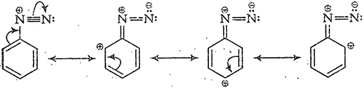
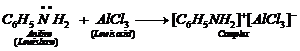 (vi) Diazonium salts of aromatic
amines are more stable than those ofaliphatic amines because these are
resonance stabilized while nosuch resonance stabilization is possible in the
corresponding diazonium salts of aliphatic amines.
(vi) Diazonium salts of aromatic
amines are more stable than those ofaliphatic amines because these are
resonance stabilized while nosuch resonance stabilization is possible in the
corresponding diazonium salts of aliphatic amines.
 (vii) Gabriel phthalimide
synthesis produces primary amines onlywithout the traces of secondary or
tertiary amines. So, this methodis preferred for the synthesis of primary
amines.
(vii) Gabriel phthalimide
synthesis produces primary amines onlywithout the traces of secondary or
tertiary amines. So, this methodis preferred for the synthesis of primary
amines.
You need to login to perform this action.
You will be redirected in
3 sec
