Answer:
In Gabriel phthalimide reaction, potassium salt of
phthalimide is formed. It reacts readily with alkyl halide to form the
corresponding alkyl derivative.
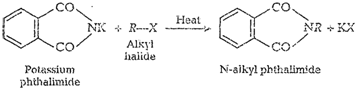 But aryl halide does not react
with potassium phthalimide. BecauseC?X bond in haloarene (alkyl halide) is
difficult to be cleaved due topartial double bond character. So, aromatic
primary amines cannot beprepared by Gabriel phthalimide synthesis.
But aryl halide does not react
with potassium phthalimide. BecauseC?X bond in haloarene (alkyl halide) is
difficult to be cleaved due topartial double bond character. So, aromatic
primary amines cannot beprepared by Gabriel phthalimide synthesis.
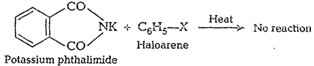
You need to login to perform this action.
You will be redirected in
3 sec
