Answer:
(i) Amines are less acidic than alcohols of comparable
molecular masses because ![]() bond is less polar than
bond is less polar than
![]() bond.
Hence, amines release
bond.
Hence, amines release ![]() ion with more
difficultly ascompared to alcohol.
(ii) Intermolecular hydrogen bonding is
present in primary amines but not in tertiary amines (H-atom absent, in amino
group) soprimary amines have higher boiling point than tertiary amines.
ion with more
difficultly ascompared to alcohol.
(ii) Intermolecular hydrogen bonding is
present in primary amines but not in tertiary amines (H-atom absent, in amino
group) soprimary amines have higher boiling point than tertiary amines.
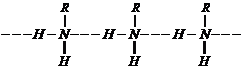 (iii) Aliphatic amines are
stronger bases than aromatic amines due to following reasons:
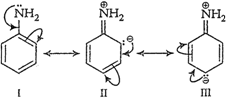
(a) Electron pair on the
nitrogen atom of aromatic amines is involved in conjugation with the
(iii) Aliphatic amines are
stronger bases than aromatic amines due to following reasons:
(a) Electron pair on the
nitrogen atom of aromatic amines is involved in conjugation with the ![]() electron
pairs of the ring as follows
electron
pairs of the ring as follows
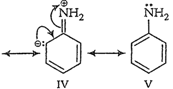 (b) Anilinium ion obtained by
accepting a proton is less stabilized by resonance.
(b) Anilinium ion obtained by
accepting a proton is less stabilized by resonance.
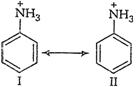 So, aniline is a weaker base than alkyl
amines, in which
So, aniline is a weaker base than alkyl
amines, in which ![]() effectincreases the
electron density on the nitrogen atom.
effectincreases the
electron density on the nitrogen atom.
You need to login to perform this action.
You will be redirected in
3 sec
