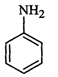
 (b)
(b) Answer:
(c)
Amines (a, b) have a stronger tendency to accept a proton and hence are
stronger Bronsted bases than phenol (c) and alcohol (d). Since phenol is more
acidic than alcohol, therefore, phenol (c) has the least tendency to accept a
proton and hence it is the weakest Bronsted base.
You need to login to perform this action.
You will be redirected in
3 sec
