Answer:
Only effective collision lead to the
formation of products. It means that collisions in which molecules collide with
sufficient kinetic energy (called threshold energy = activation energy + energy
possessed by reacting species).
And proper orientation lead to a
chemical change because it facilitates the breaking of old bonds between
(reactant) molecules and formation of the new ones i.e., in products.
e.g., formation of methanol from
bromomethane depends upon the orientation of the reactant molecules.
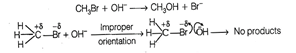
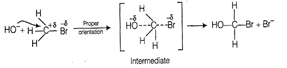 The proper orientation of
reactant; molecules leads to bond formation whereas improper orientation makes
them simply back and no products are formed.
To account for effective
collisions, another factor P (probability or steric factor) is introduced
The proper orientation of
reactant; molecules leads to bond formation whereas improper orientation makes
them simply back and no products are formed.
To account for effective
collisions, another factor P (probability or steric factor) is introduced
![]()
You need to login to perform this action.
You will be redirected in
3 sec
