Answer:
r = k [A]2
(i) When
concentration is doubled, then the new rate
r? = k [2A]2
?.(ii)
Divide (ii) by
(i)
![]()
![]()
![]() Thus, rate of
reaction becomes four times.
(ii) When
concentration of the reactant is reduced to
Thus, rate of
reaction becomes four times.
(ii) When
concentration of the reactant is reduced to ![]() , then
the new rate.
, then
the new rate.
![]() Divide (ii) by
(i)
Divide (ii) by
(i)
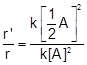
![]()
![]() Thus, rate of reaction is reduced
to one-fourth.
Thus, rate of reaction is reduced
to one-fourth.
You need to login to perform this action.
You will be redirected in
3 sec
