 (a)
(a)
Answer:
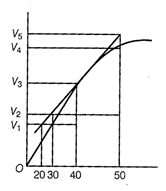
(b) Reaction occurring at smallest
time interval is known as instantaneous rate of reaction e.g., instantaneous
rate of reaction at 40 s is rate of reaction during a small interval of time
close to 40 s. Volume change during a small time interval close to 40 s i.e., 40
- 30s, 50 - 40s, 50 - 30s, 40 - 20s.
instantaneous rate of reaction
=![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c) ![]() (d)
(d) ![]()
You need to login to perform this action.
You will be redirected in
3 sec
