| Experiment | Initial concentration of [A]/molL-1 | Initial concentration of [B]/molL-1 | Initial concentration of [CJ/moll-1s-1 |
| 1. 2. 3. | 0.30 0.30 0.60 | 0.30 0.60 0.30 | 0.10 0.40 0.20 |
Answer:
(b) Rate of reaction is change in
concentration of reactant with respect to time.
![]()
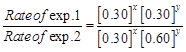
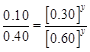
![]()
![]() Y = 2
Y = 2
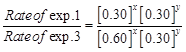
![]()
![]()
![]() i.e.,
X = 1
i.e.,
X = 1
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
