Answer:
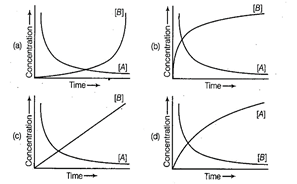
(b) A ?> 6
Concentration of reactants and products varies
exponentially w.r.t time.
(i) Concentration of reactant
(here, A) decreases exponentially w.r.t time.
(ii) Concentration of product
(here, B) increases exponentially w.r.t time new line correct graph
representing the above reaction is (b).
You need to login to perform this action.
You will be redirected in
3 sec
