Answer:
(a, c)
Distribution of kinetic energy may be
described by plotting a graph of fraction of molecules versus kinetic
energy.
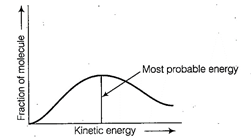 Kinetic energy of maximum
fraction of molecule is known as most probable kinetic energy. It is important
to note that with increase of temperature, peak shifts forward but downward.
This means that with increase of
temperature,
(i) most probable kinetic energy
increases.
(ii) the fractions of molecules
possessing most probable kinetic energy decreases.
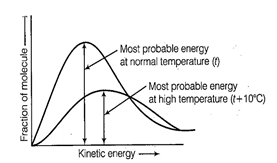
Kinetic energy of maximum
fraction of molecule is known as most probable kinetic energy. It is important
to note that with increase of temperature, peak shifts forward but downward.
This means that with increase of
temperature,
(i) most probable kinetic energy
increases.
(ii) the fractions of molecules
possessing most probable kinetic energy decreases.
You need to login to perform this action.
You will be redirected in
3 sec
