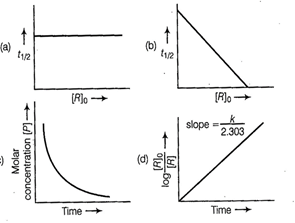
Answer:
(a, d)
For the first order reaction
![]()
![]()
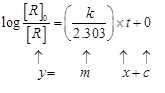 Correct plot of log
Correct plot of log ![]() can be represented by
where, slope
can be represented by
where, slope ![]() The time taken for any fraction
of the reaction to complete is independent of the initial concentration. Let,
us consider it for half of the reaction to complete.
The time taken for any fraction
of the reaction to complete is independent of the initial concentration. Let,
us consider it for half of the reaction to complete.
![]() For half-life
For half-life ![]()
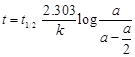
![]()
![]()
![]() is independent of initial
concentration. Hence, correct plot of
is independent of initial
concentration. Hence, correct plot of ![]() and
and ![]() can be represented by a.
can be represented by a.
You need to login to perform this action.
You will be redirected in
3 sec
