Answer:
(a, c)
During the electrolysis of dilute
sulphuric acid above given three reaction occurs each of which represents
particular reaction either oxidation half-cell reaction or reduction half cell reaction.
Oxidation half cell reactions
occur at anode are as follows
![]()
![]() Reaction having lower value of
Reaction having lower value of ![]() will undergo faster
oxidation.
Hence, oxidation of water occur
preferentially reduction half cell reaction occurs at cathode
will undergo faster
oxidation.
Hence, oxidation of water occur
preferentially reduction half cell reaction occurs at cathode
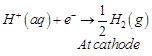 Hence, options (a) and (b) are
correct.
Hence, options (a) and (b) are
correct.
You need to login to perform this action.
You will be redirected in
3 sec
