Answer:
On
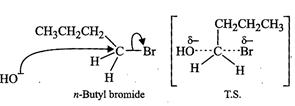
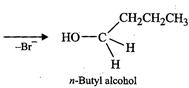
the other hand, n-butyl bromide does not undergo ionization to profluce w-butyl
carbocation because it is not stable. Therefore, it prefers to undergo reaction
by ![]() mechanism which occurs in one
step through a transition state involving nucleophilic attack of the
mechanism which occurs in one
step through a transition state involving nucleophilic attack of the ![]() ion from the back side with
simultaneous expulsion of
ion from the back side with
simultaneous expulsion of ![]() ion from
the front
side.
ion from
the front
side.
You need to login to perform this action.
You will be redirected in
3 sec
