Answer:
 (i)
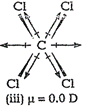
(i) ![]() (tetrachloromethane) is a symmetrical molecule so, it haszero dipole moment.
(ii) In
(tetrachloromethane) is a symmetrical molecule so, it haszero dipole moment.
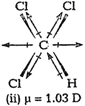
(ii) In ![]() (trichloromethane/chloroform), the resultant of twoC?CI dipole moments is opposed by the resultant of C?H andC?CI bond. Since, the latter resultant dipole moment is smallerthan the former,
(trichloromethane/chloroform), the resultant of twoC?CI dipole moments is opposed by the resultant of C?H andC?CI bond. Since, the latter resultant dipole moment is smallerthan the former, ![]() has a dipole moment = 1.03 D.
(iii) In
has a dipole moment = 1.03 D.
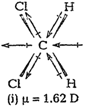
(iii) In ![]() (dichloromethane), the resultant of two C?CI dipolemoments is reinforced by the resultant of two C?H bonds. Thus,
(dichloromethane), the resultant of two C?CI dipolemoments is reinforced by the resultant of two C?H bonds. Thus,![]() has a dipole moment = 1.62 D.
Therefore,
has a dipole moment = 1.62 D.
Therefore, ![]() has the highest dipole moment amongst theabove three molecules.
Note
has the highest dipole moment amongst theabove three molecules.
Note![]() is also called carbon, tetrachloride;
is also called carbon, tetrachloride; ![]() is methylene chloride.
is methylene chloride.
You need to login to perform this action.
You will be redirected in
3 sec
