Answer:
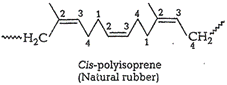
Natural rubber is cis-polyisoprene. It is obtained
bypolymerization of isoprene units at 1, 4 positions. In rubber molecule,double
bonds are located between ![]() and
and ![]() of
each isoprene unit. These cis-double bonds do not allow the polymer chain to
come closer.Therefore, only weak van der waals' forces are present. Since,
thechains are not linear, they can be stretched just like springs and
exhibitelastic properties.
of
each isoprene unit. These cis-double bonds do not allow the polymer chain to
come closer.Therefore, only weak van der waals' forces are present. Since,
thechains are not linear, they can be stretched just like springs and
exhibitelastic properties.
You need to login to perform this action.
You will be redirected in
3 sec
