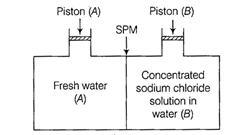
 (a) Water will move from side
(A) to side (B) if a pressure lower osmotic pressure is applied on piston (B)
(b) Water will move from side
(B) to side (A) if a pressure greater than osmotic pressure is applied on
piston (B)
(c) Water will move from side (B)
to side (A) if a pressure equal to osmotic pressure is applied on piston (B)
(d) Water will move from side
(A) to side (B) if pressure equal to osmotic pressure is applied on piston (A)
(a) Water will move from side
(A) to side (B) if a pressure lower osmotic pressure is applied on piston (B)
(b) Water will move from side
(B) to side (A) if a pressure greater than osmotic pressure is applied on
piston (B)
(c) Water will move from side (B)
to side (A) if a pressure equal to osmotic pressure is applied on piston (B)
(d) Water will move from side
(A) to side (B) if pressure equal to osmotic pressure is applied on piston (A)
Answer:
(b) We know that, if a pressure
higher than the osmotic pressure is applied on the solution, the solvent will
flow from the solution into the pure solvent through the semi-permeable
membrane. This process is called reverse osmosis.
Thus, in this case, water will move from
side (B) to side (A) if a pressure greater than osmotic pressure is applied on
piston (B).
You need to login to perform this action.
You will be redirected in
3 sec
