Answer:
Effect of
temperature. Temperature has different effect in physisorption and
chemisorption.
(i) In
Physisorption.
Adsorption is exothermic process but reverse process i.e., desorption is
endothermic. An increase in temperature will favourthe desorption process
because molecules of the adsorbate get energy and leave the surface so rate of
adsorption decreases with increase in temperature. A graph plotted between
amount
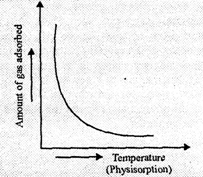
 of gas adsorbed and
temperature at a constant pressure is known as Adsorption Isobar.
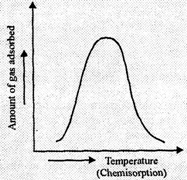
(ii) In
Chemisorption.
With initial rise in temperature, the rate of adsorption increases but with
further rise in temperature, after a certain limit, adsorption starts
decreasing. This is because an initial rise in temperature will provide the
molecules necessary activation energy for chemical bond formation so rate of
adsorption increases. At a certain temperature all the bonds are formed and now
the further increase in temperature will favourdesportion i.e., rate the
adsorption now starts decreasing.
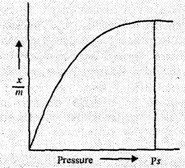
Effect of pressure.
Adsorption
of a gas by an adsorbent (solid) depends upon the pressure of the gas.
Initially the amount of gas adsorbed for a given amount of adsorbent (x/m)
increases rapidly with the increase in pressure. However, as the pressure
becomes high and almost the entire surface of the adsorbent gets saturated with
the gas, the effect of pressure becomes very small. Ultimately a stage is
reached when no more adsorption occurs even if the pressure is increased. This
stage is known as saturation Stage and pressure applied is known as saturation
pressure.
of gas adsorbed and
temperature at a constant pressure is known as Adsorption Isobar.
(ii) In
Chemisorption.
With initial rise in temperature, the rate of adsorption increases but with
further rise in temperature, after a certain limit, adsorption starts
decreasing. This is because an initial rise in temperature will provide the
molecules necessary activation energy for chemical bond formation so rate of
adsorption increases. At a certain temperature all the bonds are formed and now
the further increase in temperature will favourdesportion i.e., rate the
adsorption now starts decreasing.
Effect of pressure.
Adsorption
of a gas by an adsorbent (solid) depends upon the pressure of the gas.
Initially the amount of gas adsorbed for a given amount of adsorbent (x/m)
increases rapidly with the increase in pressure. However, as the pressure
becomes high and almost the entire surface of the adsorbent gets saturated with
the gas, the effect of pressure becomes very small. Ultimately a stage is
reached when no more adsorption occurs even if the pressure is increased. This
stage is known as saturation Stage and pressure applied is known as saturation
pressure.
You need to login to perform this action.
You will be redirected in
3 sec
