Answer:
(a) Both assertion and reason are correct statements, and
reason is the correct explanation of the assertion.
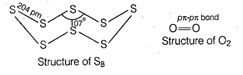
Both rhombic and mohoclinic. Sulphur exist as ![]() but oxygen exists as
but oxygen exists as ![]() , because oxygen forms
, because oxygen forms ![]() multiple bond due to its small
size and small bond length. But
multiple bond due to its small
size and small bond length. But ![]() bonding is not
possible in sulphur due to its bigger size as compared to oxygen.
bonding is not
possible in sulphur due to its bigger size as compared to oxygen.
You need to login to perform this action.
You will be redirected in
3 sec
