Answer:
(a) Compounds having same value of total number of electrons
are known as isoelectronic.
For![]()
![]() Total number of electrons Total number of
electrons
= 6 + 8 x 3 + 2 =7+ 8 x 3 +
1
= 6 + 24 + 2 =7 + 25
= 32 . =32
Hence,
Total number of electrons Total number of
electrons
= 6 + 8 x 3 + 2 =7+ 8 x 3 +
1
= 6 + 24 + 2 =7 + 25
= 32 . =32
Hence, ![]() and
and ![]() are isoelectronic. These two ions
have similar structure so they are isostructural.
are isoelectronic. These two ions
have similar structure so they are isostructural.
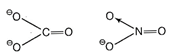 Both have triangular planar structure as in both the
species carbon and nitrogen are sp2 hybridised. Hence, (a) is the
correct choice.
Both have triangular planar structure as in both the
species carbon and nitrogen are sp2 hybridised. Hence, (a) is the
correct choice.
You need to login to perform this action.
You will be redirected in
3 sec
