Answer:
(a-) True statement is that single N ? N bond is weaker than
the single P. ? P bond. This is
why phosphorous show allotropy but nitrogen does not.
(i) PH3 acts as a ligand in the formation of
coordination compound due to presence of lone pair of electrons.
(ii) NO2 is paramagnetic in nature due to
presence of one unpaired electron.
Structure of NO2 is
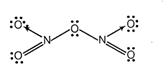
![]() (iii) Covalency of nitrogen in
(iii) Covalency of nitrogen in ![]() is
4.
is
4.
You need to login to perform this action.
You will be redirected in
3 sec
