Answer:
(b,
d)
Structure of P4 molecule can be represented as
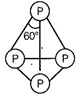 It has total four lone pairs of electrons situated at each
P - atom.
It has six P?P single bond.
It has total four lone pairs of electrons situated at each
P - atom.
It has six P?P single bond.
You need to login to perform this action.
You will be redirected in
3 sec
