Answer:
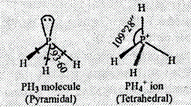
P in both PH3
and PH4+ is ![]() hybridized. Due to
the absence of lone pair-bond pair repulsion and presence of four identical
bond pair-bond pair interactions, PH4+ assumes
tetrahedral geometry with a bond angle to 109° 28'. But PH3 has
three bond pairs and one lone pair around P. Due to greater lone pair-bond pair
repulsion than bond pair-bond pair repulsion, the tetrahedral angle decreases
from 109° 28' to 93.6°. As a result, PH3 is pyramidal.
hybridized. Due to
the absence of lone pair-bond pair repulsion and presence of four identical
bond pair-bond pair interactions, PH4+ assumes
tetrahedral geometry with a bond angle to 109° 28'. But PH3 has
three bond pairs and one lone pair around P. Due to greater lone pair-bond pair
repulsion than bond pair-bond pair repulsion, the tetrahedral angle decreases
from 109° 28' to 93.6°. As a result, PH3 is pyramidal.
You need to login to perform this action.
You will be redirected in
3 sec
