Answer:
It
has trigonal bipyramidal geometry, in which two Cl atoms occupy axial position
while three occupy equatorial positions. All five P?Cl bonds are not identical.
There are two types of bond lengths (i) Axial bond lengths (ii) Equatorial bond
lengths
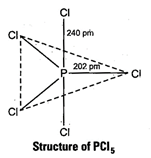 Thus, difference in bond length is due to fact that axial
bond pairs suffer more repulsion as compared to equatorial bond pairs.
Thus, difference in bond length is due to fact that axial
bond pairs suffer more repulsion as compared to equatorial bond pairs.
You need to login to perform this action.
You will be redirected in
3 sec
