Answer:
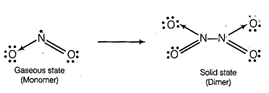
In
gaseous state, NO2 exists as a monomer which has one unpaired
electron but in solid state, it dimerises to ![]() so
no unpaired electron left. Therefore, NO2 is paramagnetic in gaseous
state but diamagnetic in solid state.
so
no unpaired electron left. Therefore, NO2 is paramagnetic in gaseous
state but diamagnetic in solid state.
You need to login to perform this action.
You will be redirected in
3 sec
