Answer:
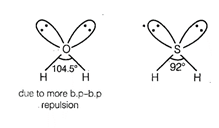
Bond
angle of ![]() (1-1?0?H = 104.5°) is larger than
that of
(1-1?0?H = 104.5°) is larger than
that of ![]() (H?S?H = 92°) because oxygen is
more electronegative than sulphur therefore, bond pair electron of 0?H bond
will be closer to oxygen and there will be more bond pair?bond pair repulsion between
bond pairs of two 0?H bonds.
(H?S?H = 92°) because oxygen is
more electronegative than sulphur therefore, bond pair electron of 0?H bond
will be closer to oxygen and there will be more bond pair?bond pair repulsion between
bond pairs of two 0?H bonds.
You need to login to perform this action.
You will be redirected in
3 sec
