Answer:
Equations for the reactions
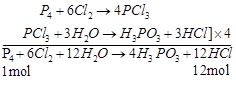
![]()
![]()
![]() 124 g of white phosphorus
produces HCI = 438 g
124 g of white phosphorus
produces HCI = 438 g
![]() 62 g of white phosphorus
will produces
62 g of white phosphorus
will produces
![]()
You need to login to perform this action.
You will be redirected in
3 sec
