-
Silver atom has completely filled d-orbitals (4d10) in its ground state. How can you say that it is a transition element?
View Answer play_arrow
-
In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest i.e. 126 kJ mol–1. Why?
View Answer play_arrow
-
Which is the 3d series of the transition metals exhibits the largest number of oxidation states and why?
View Answer play_arrow
-
The 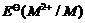 value
for copper is positive (+0.34 V). What is possible the reason for this?
value
for copper is positive (+0.34 V). What is possible the reason for this?
View Answer play_arrow
-
How would you account for irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
View Answer play_arrow
-
Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
View Answer play_arrow
-
Which is a stronger reducing agent, Cr2+ or Fe2+ and why?
View Answer play_arrow
-
Calculate
the 'spin only' magnetic moment of 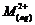 ion (Z
= 27).
ion (Z
= 27).
View Answer play_arrow
-
Explain why Cu+ ion is not stable in aqueous solutions?
View Answer play_arrow
-
Actinoid contraction is greater from element to than lanthanoid contraction. Why?
View Answer play_arrow
-
Write
down the electronic configuration of
(a)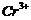 (b)
(b) 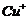 (c)
(c) 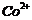 (d)
(d)
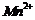 (e)
(e) 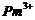 (f)
(f)
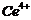 (g)
(g) 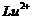 (h)
(h)
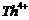
View Answer play_arrow
-
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state ?
View Answer play_arrow
-
Explain briefly how +2 oxidation state becomes more and more stable in the first half of the first row transition elements with increasing atomic number.
View Answer play_arrow
-
To what extent do the electronic configurations decide the stability or oxidation states in the first series of the transition elements? Illustrate your answer with an example.
View Answer play_arrow
-
What must be the stable oxidation state of the transition elements with the following electronic configuration in the ground states or their atoms: 3d3, 3d5, 3d8, 3d4?
View Answer play_arrow
-
Name the oxometal anions in the first transition series of transition metals in which the metal exhibits oxidation state equal to its group number.
View Answer play_arrow
-
What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
View Answer play_arrow
-
What are the characteristics of transition elements and why are they called transition elements? Which of the d-block elements may not be regarded as the transition elements?
View Answer play_arrow
-
In what way is the electronic configuration of the transition elements different from that of the non-transation elements?
View Answer play_arrow
-
What are the different oxidation states exhibited by the lanthanoids?
View Answer play_arrow
-
Explain giving reasons :
(a) Transition metals and many of their compounds show paramagnetic behaviour.
(b) Transition metals have high enthalpy of atomisation.
(c) The transition metals generally form coloured compounds.
(d) Transitional metals and their many compounds act as good catalysts.
View Answer play_arrow
-
What are interstitial compounds? Why are such compounds well known for transition metals?
View Answer play_arrow
-
How is variability in oxidation states of transition metals different from that of non-transition metals? Illustrate with examples.
View Answer play_arrow
-
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
View Answer play_arrow
-
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with :
(a) iodide (b) iron (iii) solution (c) H2S.
View Answer play_arrow
-
Describe the preparation of potassium permanganate. How does the acidified permanganate solution reacts with :
(i) iron (ii) ions (iii) SO2 (iv) oxalic acid ?
Write the ionic equations for the reactions.
View Answer play_arrow
-
For M2+/M
and M3 + /M2+ systems, the 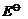 values of some
metals are given :
values of some
metals are given :
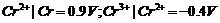
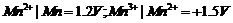
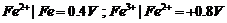 Use this data to
comment upon:
(a) The stability
of Fe3+ in acid solution as compared to that of Cr3+ or
Mn3+.
(b) The ease with
which iron can be oxidised as compared to the similar process for either
chromium or manganese metal.
Use this data to
comment upon:
(a) The stability
of Fe3+ in acid solution as compared to that of Cr3+ or
Mn3+.
(b) The ease with
which iron can be oxidised as compared to the similar process for either
chromium or manganese metal.
View Answer play_arrow
-
Predict which of the following will be coloured in aqueous solution?
Ti3+, V3+, Cu+, Sc3+, Mn2+ , Fe3+ and Co2+
Give reason for each.
View Answer play_arrow
-
Compare the stability of +2 oxidation state for the elements of the first transition series.
View Answer play_arrow
-
Compare the chemistry of actinoids with that of lanthanoids with special reference to
(i) Electronic configuration (ii) Atomic and ionic sizes
(iii) Oxidation state (iv)Chemical reactivity.
View Answer play_arrow
-
How would you account for the following
(a) Of the d4 species, Cr2+ is strongly reducing while Mn3+ is strongly oxidising in nature.
(b) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
(c) The d1 configuration is very unstable in ions.
View Answer play_arrow
-
What are disproportionation reactions? Give two examples.
View Answer play_arrow
-
Which metal in the first transition metal series exhibits +1 oxidation state most frequently and why?
View Answer play_arrow
-
Calculate the number of unpaired electrons in the following gaseous ions :
Mn3+, Cr3+, V3+ and Ti3+
Which one of these is most stable in aqueous solution?
View Answer play_arrow
-
Give example and suggest reasons for the following features of transition metal chemistry:
(a) The lowest oxide of the transition metal is basic while the highest is acidic.
(b) A transition metal exhibits higher oxidation states in oxides and fluorides.
(c) The highest oxidation state is exhibited in oxoanions of a metal.
View Answer play_arrow
-
Indicate the steps in the preparation of:
(a) K2Cr2O7 from chromite ore.
(b) KMnO4 from pyrolusite ore.
View Answer play_arrow
-
What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
View Answer play_arrow
-
What are inner transition elements? Decide which or the following atomic numbers belong to inner transition elements : 29, 59, 74, 95, 102, 104
View Answer play_arrow
-
The chemistry of actinoid elements is not so smooth as that of lanthanoids. Justify this statement by giving some examples from the oxdiation states of these elements.
View Answer play_arrow
-
Which is the last element in the series of actinoids? Write the electronic configuration of the element. Comment upon the possible oxidation state.
View Answer play_arrow
-
Use Hand's rule to derive the electron configuration of Ce3+ ion and calculate it, magnetic moment on the basis of spin-only formula.
View Answer play_arrow
-
Name the members of lanthanoid series which exhibit +4 oxidation states and those which exhibit +2 oxidation states. Try to correlate this type of behaviour with the electronic configuration of these elements.
View Answer play_arrow
-
Compare the chemistry of the actinoids with that of lanthanoids with reference to :
(i) Electronic configuration
(ii) Oxidation states
(iii) Chemical reactivity.
View Answer play_arrow
-
Write the electronic configuration of the elements with atomic numbers 61, 91, 101, 109.
View Answer play_arrow
-
Compare the general characteristics of the first series of transition metals with those of those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points :
(i) electronic configurations, (ii) oxidation states, (iii) ionisation enthalpies and (iv) atomic sizes.
View Answer play_arrow
-
Write down the number or 3d electrons in each or the following ions :Ti2+, V2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+.Indicate how would you expect the rive 3d orbitals to be occupied for these hydrated ions (octahedral).
View Answer play_arrow
-
Comment on the statement that the elements of the first transition series possess many properties different from those of heavier transition elements
View Answer play_arrow
-
What can be inferred from the magnetic moment of the following complex species
Example Magnetic moment (BM)
K4[Mn(CN)6] 2.2
[Fe(H2O)6]2+ 5.3
K2 [MnCl4] 5.9
View Answer play_arrow
-
question_answer49)
Electronic configuration of
a transition element X in +3 oxidation state is 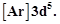 what
is its atomic number?
(a) 25
(b) 26 (c) 27 (d) 24
what
is its atomic number?
(a) 25
(b) 26 (c) 27 (d) 24
View Answer play_arrow
-
question_answer50)
The electronic configuration
of 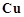 (II) is where
(II) is where 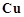 as that of
as that of 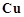 (l) is
(l) is 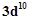 Which of the following
is correct?
(a) Cu (II) is more
stable
(b) Cu (ll) is less stable
(c) Cu (l) and Cu (ll)
are equally stable
(d) Stability of Cu
(l) and Cu(ll) depends on nature of copper salts
Which of the following
is correct?
(a) Cu (II) is more
stable
(b) Cu (ll) is less stable
(c) Cu (l) and Cu (ll)
are equally stable
(d) Stability of Cu
(l) and Cu(ll) depends on nature of copper salts
View Answer play_arrow
-
question_answer51)
Metallic radii of some
transition elements are given below. Which of these elements will have highest
density?
|
Element
|
Fe
|
Co
|
Ni
|
Cu
|
|
Metallic radii/pm
|
126
|
125
|
125
|
128
|
(a) Fe
(b)Ni (c) Co (d) Cu
View Answer play_arrow
-
question_answer52)
Generally, transition
elements form coloured salts due to the presence of unpaired electrons. Which
of the following compounds will be coloured in solid state?
(a)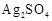 (b)
(b) 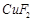 (c)
(c) 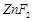 (d)
(d) 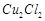
View Answer play_arrow
-
question_answer53)
On addition of small amount
of 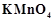
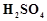 to
concentrated H 2804, a green oily compound is obtained which is highly explosive
in nature. Identify the compound from the following.
(a)
to
concentrated H 2804, a green oily compound is obtained which is highly explosive
in nature. Identify the compound from the following.
(a)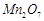 (b)
(b) 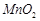 (c)
(c) 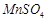 (d)
(d) 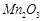
View Answer play_arrow
-
question_answer54)
The magnetic, nature of
elements depends on the presence of unpaired electrons. Identify the
configuration of transition element, .which shows highest magnetic moment.
(a) 3d7
(b) 3d5
(c) 3d8
(d) 3d2
View Answer play_arrow
-
question_answer55)
Which of the following
oxidation state is common for all lanthanoids?
(a) +2 (b)
+3 (c) +4 (d) +5
View Answer play_arrow
-
question_answer56)
Which of the following
reactions are disproportionation reactions?
(i)  (ii)
(ii) 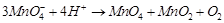 (iii)
(iii) 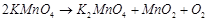 (iv)
(iv) 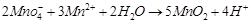 (a)
(i) (b) (i), (ii) and (iii)
(c) (ii), (iii) and
(iv) (d) (i) and (iv)
(a)
(i) (b) (i), (ii) and (iii)
(c) (ii), (iii) and
(iv) (d) (i) and (iv)
View Answer play_arrow
-
question_answer57)
When 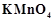 solution is added to oxalic add
solution, the decolourisation is slow in the beginning but becomes
instantaneous after some time because
(a)
solution is added to oxalic add
solution, the decolourisation is slow in the beginning but becomes
instantaneous after some time because
(a) 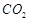 is formed as the product
(b) reaction
is exothermic
(c)
is formed as the product
(b) reaction
is exothermic
(c) 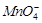 catalyses the reaction
(d)
catalyses the reaction
(d) 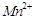 acts as autocatalyst
acts as autocatalyst
View Answer play_arrow
-
question_answer58)
There are 14 elements in
actinoid series. Which of the following elements does not belong to this
series?
(a) U
(b) Np (c) Tm (d) Fm
View Answer play_arrow
-
question_answer59)
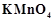 acts
as an oxidising agent in acidic medium. The number of moles of
acts
as an oxidising agent in acidic medium. The number of moles of 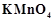 that will be needed to react
with one mole of sulphide ions in acidic solution is
(a)
that will be needed to react
with one mole of sulphide ions in acidic solution is
(a) 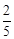 (b)
(b)
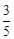 (c)
(c) 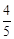 (d)
(d)
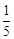
View Answer play_arrow
-
question_answer60)
Which of the following is
amphoteric oxide?
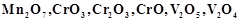 (a)
(a) 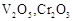 (b)
(b) 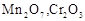 (c)
(c) 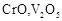 (d)
(d) 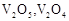
View Answer play_arrow
-
question_answer61)
Gadolinium belongs to 4f series.
Its atomic number is 64. Which of the following is the correct electronic
configuration of gadolinium?
(a) 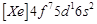 (b)
(b) 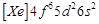 (c)
(c) 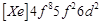 (d)
(d) 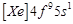
View Answer play_arrow
-
question_answer62)
Interstitial compounds are
formed when small atoms are trapped inside the crystal lattice of metals. Which
of the following is not the characteristic property of interstitial compounds?
(a)They have high
melting points in comparison to pure metals
(b) They are
very hard
(c) They
retain metallic conductivity
(d) The are
chemically very reactive
View Answer play_arrow
-
question_answer63)
The magnetic moment is
associated with its spin angular momentum and orbital angular momentum. Spin only
magnetic moment value of 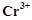 ion is
(a) 2.87BM (b)
3.87 BM
(c) 3.47BM
(d) 3.57BM
ion is
(a) 2.87BM (b)
3.87 BM
(c) 3.47BM
(d) 3.57BM
View Answer play_arrow
-
question_answer64)
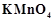 acts
as an oxidising agent in alkaline medium. When alkaline KMn04 is treated with
KI, iodide ion is oxidised to..........
(a)
acts
as an oxidising agent in alkaline medium. When alkaline KMn04 is treated with
KI, iodide ion is oxidised to..........
(a) 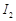 (b)
(b)
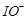 (c)
(c) 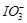 (d)
(d)
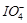
View Answer play_arrow
-
question_answer65)
Which of the following
statements is not correct?
(a) Copper liberates
hydrogen from acids
(b) In its
higher oxidation states, manganese forms stable compounds with oxygen and
fluorine
(c) 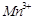 and
and 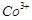 are
oxidising agents in aqueous solution
(d)
are
oxidising agents in aqueous solution
(d) 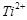 and
and 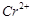 are
reducing agents in aqueous solution
are
reducing agents in aqueous solution
View Answer play_arrow
-
question_answer66)
When acidified 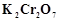 solution is added to
solution is added to 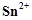 salt then
salt then 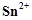 changes to
(a)
changes to
(a) 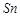 (b)
(b)
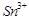 (c)
(c) 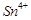 (d)
(d) 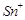
View Answer play_arrow
-
question_answer67)
Highest oxidation state of
manganese in fluoride is +4 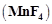 but highest
oxidation state in oxides is +7
but highest
oxidation state in oxides is +7 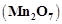 because
(a) fluorine is more
electronegative than oxygen
(b) fluorine
does not possess d orbitals
(c) fluorine
stabilises lower oxidation state
(d) in covalent
compounds, fluorine can form single bond only while oxygen forms double bond
because
(a) fluorine is more
electronegative than oxygen
(b) fluorine
does not possess d orbitals
(c) fluorine
stabilises lower oxidation state
(d) in covalent
compounds, fluorine can form single bond only while oxygen forms double bond
View Answer play_arrow
-
question_answer68)
Although zirconium belongs to 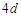 transition series and hafniun to
transition series and hafniun to 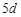 transition series even then they
show similar physical and chemical properties because..............
(a) both belong to d
- block
(b) both
have same number of electrons
(c) both
have similar atomic radius
(d) both belong to
the same group of the Periodic Table
transition series even then they
show similar physical and chemical properties because..............
(a) both belong to d
- block
(b) both
have same number of electrons
(c) both
have similar atomic radius
(d) both belong to
the same group of the Periodic Table
View Answer play_arrow
-
question_answer69)
Why is HCl not used to make
the medium acidic in oxidation reactions of 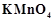 in
acidic medium?
(a) Both HCl and
in
acidic medium?
(a) Both HCl and 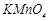 act as oxidising agents
(b)
act as oxidising agents
(b) 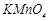 oxidises HCl into
oxidises HCl into 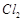 which is also an oxidising agent
(c)
which is also an oxidising agent
(c) 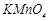 is a weaker oxidising agent than
HCl
(d)
is a weaker oxidising agent than
HCl
(d) 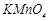 acts as a reducing agent in the
presence of HCl
acts as a reducing agent in the
presence of HCl
View Answer play_arrow
-
question_answer70)
Generally transition
elements and their salts are coloured due to the presence of unpaired electrons
in metal ions. Which of the following compounds are coloured?
(a) 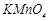 (b)
(b) 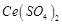 (c)
(c) 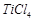 (d)
(d) 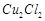
View Answer play_arrow
-
question_answer71)
Transition elements show
magnetic moment due to spin and orbital motion of electrons. Which of
the following metallic ions have almost same spin only magnetic moment?
(a) 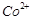 (b)
(b) 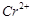 (c)
(c) 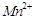 (d)
(d) 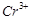
View Answer play_arrow
-
question_answer72)
In the form of dichromate,
Cr (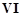 ) is a strong oxidising agent in
acidic medium but Mo (VI) in
) is a strong oxidising agent in
acidic medium but Mo (VI) in 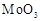
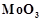 and W() in
and W() in 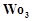 are not because
(a) Cr (VI) is more
stable than Mo(
are not because
(a) Cr (VI) is more
stable than Mo(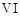 ) and W(VI).
(b) Mo (VI)
and W (VI) are more stable than Cr (VI).
(c) Higher
oxidation states of heavier members of group 6 of transition series are more
stable.
(d) Lower oxidation
states of heavier members of group-6 of transition series are more stable.
) and W(VI).
(b) Mo (VI)
and W (VI) are more stable than Cr (VI).
(c) Higher
oxidation states of heavier members of group 6 of transition series are more
stable.
(d) Lower oxidation
states of heavier members of group-6 of transition series are more stable.
View Answer play_arrow
-
question_answer73)
Which of the following
actinoids show oxidation states upto +7?
(a) Am (b)
Pu
(c) U (d)
Np
View Answer play_arrow
-
question_answer74)
General electronic
configuration of actinoids is 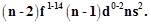 Which
of the following actinoids have one electron in 6d orbital?
(a) U (Atomic
number. 92)
(b) Np
(Atomic number. 93)
(c) Pu
(Atomic number. 94)
(d) Am (Atomic
number. 95)
Which
of the following actinoids have one electron in 6d orbital?
(a) U (Atomic
number. 92)
(b) Np
(Atomic number. 93)
(c) Pu
(Atomic number. 94)
(d) Am (Atomic
number. 95)
View Answer play_arrow
-
question_answer75)
Which of the following
lanthanoids show +2 oxidation state besides the characteristic oxidation state
+3 of lanthanoids?
(a) Ce (b)
Eu
(c) Yb (d)
Ho
View Answer play_arrow
-
question_answer76)
Which of the following ions
show higher spin only magnetic moment value?
(a) 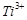 (b)
(b)
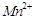 (c)
(c) 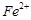 (c)
(c)
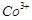
View Answer play_arrow
-
question_answer77)
Transition elements form
binary compounds with halogens. Which of the following elements will form Mf^
type compounds?
(a) Cr (b)
Co
(c) Cu (d)
Ni
View Answer play_arrow
-
question_answer78)
Which of the following will
not act as oxidising agents?
(a) 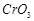 (b)
(b)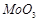 (c)
(c) 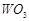 (d)
(d)
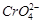
View Answer play_arrow
-
question_answer79)
Although +3 is the
characteristic oxidation state for lanthanoids but cerium also shows+4
oxidation state because
(a) it has variable
ionisation enthalpy
(b) it has a
tendency to attain noble gas configuration
(c) it has a
tendency to attain 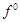 configuration
(d) it resembles
configuration
(d) it resembles 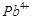
View Answer play_arrow
-
question_answer80)
Why does copper not replace
hydrogen from acids?
View Answer play_arrow
-
question_answer81)
Why 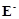 values for Mn, Ni and Zn are
more negative than expected?
values for Mn, Ni and Zn are
more negative than expected?
View Answer play_arrow
-
question_answer82)
Why first ionisation
enthalpy of Cr is lower than that of Zn?
View Answer play_arrow
-
question_answer83)
Transition elements show
high melting points. Why?
View Answer play_arrow
-
question_answer84)
When 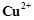 ion is treated with KI, a white
precipitate is formed. Explain the reaction with the help of chemical equation.
ion is treated with KI, a white
precipitate is formed. Explain the reaction with the help of chemical equation.
View Answer play_arrow
-
question_answer85)
Out of 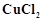 and
and 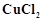 ,
which is more stable and why?
,
which is more stable and why?
View Answer play_arrow
-
question_answer86)
When a brown compound. of
manganese (A) is treated with HCL it gives a gas (B). The gas taken in excess,
reacts with 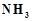 to give an explosive
compound (C) Identify compounds A, B and C.
to give an explosive
compound (C) Identify compounds A, B and C.
View Answer play_arrow
-
question_answer87)
Although fluorine is more
electronegative than oxygen, but the ability of oxygen to stabilise higher
oxidation states exceeds that of fluorine. Why?
View Answer play_arrow
-
question_answer88)
Although 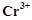 and
and 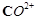 ions
have same number of unpaired electrons but the magnetic moment of
ions
have same number of unpaired electrons but the magnetic moment of 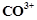 is 3.87 BM and that of
is 3.87 BM and that of 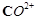 is 4.87 BM, Why?
is 4.87 BM, Why?
View Answer play_arrow
-
question_answer89)
lonisation enthalpies of Ce,
Pr and Nd are higher than Th, Pa and U. Why?
View Answer play_arrow
-
question_answer90)
Although Zr belongs to 4d
and Hf belongs to 5d transition series but it is quite difficult to separate
them. Why?
View Answer play_arrow
-
question_answer91)
Although +3 oxidation states
is the characteristic oxidation state of lanthanoids but cerium shows +4
oxidation state also. Why?
View Answer play_arrow
-
question_answer92)
Explain, why does colour of 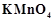 disappear when oxalic acid is
added to its solution in acidic medium?
disappear when oxalic acid is
added to its solution in acidic medium?
View Answer play_arrow
-
question_answer93)
When orange solution
containing 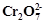 ion is treated with
an alkali, a yellow solution is formed and when
ion is treated with
an alkali, a yellow solution is formed and when 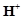 ions
are added to yellow solution, an orange solution is obtained. Explain why does
this happen?
ions
are added to yellow solution, an orange solution is obtained. Explain why does
this happen?
View Answer play_arrow
-
question_answer94)
A solution of 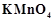 on reduction yields either a
colourless solution or a brown precipitate or a green solution depending on pH
of the solution.
What different
stages of the reduction do these represent and how are they carried out?
on reduction yields either a
colourless solution or a brown precipitate or a green solution depending on pH
of the solution.
What different
stages of the reduction do these represent and how are they carried out?
View Answer play_arrow
-
question_answer95)
The second and third rows of
transition elements resemble each other much more than they resemble the first
row. Explain, why?
View Answer play_arrow
-
question_answer96)
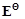 of
Cu is +0.34V while that of Zn is -0.76 V. Explain.
of
Cu is +0.34V while that of Zn is -0.76 V. Explain.
View Answer play_arrow
-
question_answer97)
The halides of transition
elements become more covalent with increasing oxidation state of the metal.
Why?
View Answer play_arrow
-
question_answer98)
While filling up of
electrons in the atomic orbitals, the As orbital is filled before the 3d
orbital but reverse happens during the ionisation of the atom. Explain why?
View Answer play_arrow
-
question_answer99)
Reactivity of transition
elements decreases almost regularly from Se to Cu. Explain.
View Answer play_arrow
-
question_answer100)
Match the catalysts given in
Column I with the processes given in Column II.
|
|
Column I
(Catalyst)
|
Column II
(Process)
|
|
A.
|
Ni in the presence of
hydrogen
|
1. Ziegler-Natta catalyst
|
|
B.
|
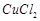
|
2. Contact process
|
|
C
|
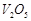
|
3. Vegetable oil to ghee
|
|
D.
|
Finely divided iron
|
4. Sandmeyer reaction
|
|
E.
|
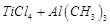
|
5. Haber's process
|
|
|
|
6. Decomposition of 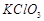
|
View Answer play_arrow
-
question_answer101)
Match the compounds/elements
given in Column I with uses given in Column II.
|
Column 1
(Compound/element)
|
Column 11 (Use)
|
|
A. Lanthanoid oxide
B. Lanthanoid
C. Misch metall
D. Magnesium based alloy is constituent of
E. Mixed oxides of lanthanoids are employed
|
1. Production of iron alloy
2. Television screen
3. Petroleum cracking 4. Lanthanoid metal + iron
5. Bullets
6. X-ray screen
|
View Answer play_arrow
-
question_answer102)
Match the properties given
in Column I with the metals given in Column II.
|
Column 1
(Property)
|
Column 11
(Metal)
|
|
A. An element which can show+8 oxidation state
B. 3d block element that can show upto +7
oxidation state
C. 3d block element with highest melting point
|
1. Mn
2. Cr
3. Os
4. Fe
|
View Answer play_arrow
-
question_answer103)
Match the statements given
in Column I with the oxidation states given in Column II.
|
|
Column 1
|
Column 11
|
|
A.
|
Oxidation state of Mn in 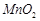 is is
|
1. +2
|
|
B.
|
Most stable oxidation state of Mn is
|
2. +3
|
|
C.
|
Most stable oxidation state of Mn in oxides is
|
3. +4
|
|
D.
|
Characteristic oxidation state of lanthanoids is
|
4. +5
|
|
|
|
5. +7
|
View Answer play_arrow
-
question_answer104)
Match the solutions given in
Column I and the colours given in Column II.
|
Column 1
(Aqueous solution of salt)
|
Column 11
(Colour)
|
A. 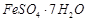
|
1. Green
|
B. 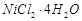
|
2. Light pink
|
C. 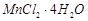
|
3. Blue
|
D. 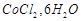
|
4. Pale green
|
E. 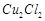
|
5. Pink
|
|
|
6. Colourless
|
View Answer play_arrow
-
question_answer105)
Match the property given in
Column I with the element given in Column II.
|
|
Column I
(Property)
|
Column II
(Element)
|
|
A.
|
Lanthanoid which shows + 4 oxidation state
|
1. Pm
|
|
B.
|
Lanthanoid which can show +2 oxidation state
|
2. Ce
|
|
C.
|
Radioactive lanthanoid
|
3. Lu
|
|
D.
|
Lanthanoid which has 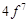 electronic electronic
|
4. Eu
|
|
|
configuration in +3 oxidation state
|
|
|
E.
|
Lanthanoid which has 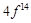 electronic
configuration in +3 oxidation state electronic
configuration in +3 oxidation state
|
5. Gd
|
|
|
|
6. Dy
|
View Answer play_arrow
-
question_answer106)
Match the properties given in
Column I with the metals given in Column II.
|
Column 1 (Property)
|
Column 11 (Metal)
|
A. Element with highest second ionisation
enthalpy
B. Element with highest third ionisation enthalpy
C. M in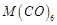 D. Element with highest heat of atomisation
D. Element with highest heat of atomisation
|
1. Col
2. Cr
3. Cu,
4. Zn
5. Ni
|
View Answer play_arrow
-
question_answer107)
Assertion (A) 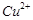 iodide is not known.
Reason (R)
iodide is not known.
Reason (R) 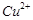 oxidises I" to iodine.
oxidises I" to iodine.
View Answer play_arrow
-
question_answer108)
Assertion (A) Separation
of Zr and Hf is difficult.
Reason (R)
Because Zr and Hf lie in the same group of the Periodic Table.
View Answer play_arrow
-
question_answer109)
Assertion (A) Actinoids
form relatively less stable complexes as compared to lanthanoids.
Reason (R)
Actinoids can utilise their 5f orbitals a longwith 6d orbitals in bonding but
lanthanoids do not use their 4/ orbital for bonding.
View Answer play_arrow
-
question_answer110)
Assertion (A) Cu cannot
liberate hydrogen from acids.
Reason (R)
Because it has positive electrode potential.
View Answer play_arrow
-
question_answer111)
Assertion (A) The
highest oxidation state of osmium is +8.
Reason (R)
Osmium is a 5d-block element.
View Answer play_arrow
-
question_answer112)
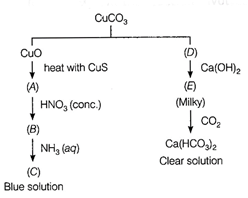
Identify A to f and also
explain the reaction involved.
View Answer play_arrow
-
question_answer113)
When a chromite ore (A) is
fused with sodium carbonate in free excess of air and the product is dissolved
in water, a yellow solution of compound (B) is obtained. After treatment of
this yellow solution with sulphuric acid, compound (C) can be crystallised from
the solution.
When compound (C)
is treated with KCl, orange crystals of compound (D) crystallise out. Identify A
to D and also explain the reactions.
View Answer play_arrow
-
question_answer114)
When an oxide of manganese (A)
is fused with KOH in the presence of an oxidising agent and dissolved in water,
it gives a dark green solution of compound (B). Compound (B) disproportionates
in neutral or acidic solution to give purple compound (C) An alkaline solution
of compound (C) oxidises potassium iodide solution to a compound (D) and
compound
(A) is
also formed. Identify compounds A to D and also explain the reactions involved.
View Answer play_arrow
-
question_answer115)
On the basis of lanthanoid
contraction, explain the following:
(i) Nature of
bonding in 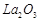 and
and 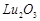 .
(ii) Trends
in the stability of oxo salts of lanthanoids from La to Lu.
(iii) Stability
of the complexes of lanthanoids.
(iv) Radii
of 4cf and 5d block elements.
(v) Trends in acidic
character of lanthanoid oxides.
.
(ii) Trends
in the stability of oxo salts of lanthanoids from La to Lu.
(iii) Stability
of the complexes of lanthanoids.
(iv) Radii
of 4cf and 5d block elements.
(v) Trends in acidic
character of lanthanoid oxides.
View Answer play_arrow
-
question_answer116)
(a) Answer the following
questions
(i) Which element
of the first transition series has highest second ionisation enthalpy?
(ii) Which
element of the first transition series has highest third ionisation enthalpy?
(iii)
Which element of the first transition series has lowest enthalpy of atomisation?
(b)
Identify the metal and justify your answer.
(i) Carbonyl
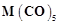 (ii)
(ii) 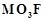
View Answer play_arrow
-
question_answer117)
Mention the type of
compounds formed when small atoms like H, C and N get trapped inside the
crystal lattice of transition metals. Also give physical and chemical
characteristics of these compounds.
View Answer play_arrow
-
question_answer118)
(a) Transition metals can
act as catalysts because these can change their oxidation state. How does Fe
(III) catalyse the reaction between iodide and persulphate ions?
(b) Mention any
three processes where transition metals act as catalysts.
View Answer play_arrow
-
question_answer119)
A violet compound of
manganese (A) decomposes on heating to liberate oxygen and compounds (B) and
(C) of manganese are formed. Compound (C) reacts with KOH in the presence of
potassium nitrate to give compound (B). On heating compound (C) with cone. 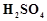 and
and 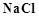 ,
chlorine gas is liberated and a compound (D) of manganese alongwith other
products is formed. Identify compounds A to D and also explain the reactions
involved.
,
chlorine gas is liberated and a compound (D) of manganese alongwith other
products is formed. Identify compounds A to D and also explain the reactions
involved.
View Answer play_arrow
![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c) ![]() (d)
(d) ![]()
![]() solution is added to
solution is added to ![]() salt then
salt then ![]() changes to
(a)
changes to
(a) ![]() (b)
(b)
![]() (c)
(c) ![]() (d)
(d) ![]()
![]() (b)
(b)![]() (c)
(c) ![]() (d)
(d)
![]()
![]() values for Mn, Ni and Zn are
more negative than expected?
values for Mn, Ni and Zn are
more negative than expected?
![]() and
and ![]() ,
which is more stable and why?
,
which is more stable and why?
![]() of
Cu is +0.34V while that of Zn is -0.76 V. Explain.
of
Cu is +0.34V while that of Zn is -0.76 V. Explain.
![]() iodide is not known.
Reason (R)
iodide is not known.
Reason (R) ![]() oxidises I" to iodine.
oxidises I" to iodine.

