Answer:
![]() line
in Balmer series corresponds to transition from n = 5 to n = 2. Therefore,
electron in ground state (n = 1), must be raised first to the state (n = 5).
Energy required for this purpose
line
in Balmer series corresponds to transition from n = 5 to n = 2. Therefore,
electron in ground state (n = 1), must be raised first to the state (n = 5).
Energy required for this purpose
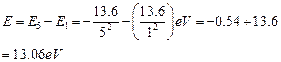 If the angular momentum of the system
is conserved, then angular momentum of photon emitted = change in angular
momentum of electron
If the angular momentum of the system
is conserved, then angular momentum of photon emitted = change in angular
momentum of electron
![]()
You need to login to perform this action.
You will be redirected in
3 sec
