Answer:
In hydrogen atom,
one electron (of mass me) revolves around one proton (of mass M) Reduced
mass for hydrogen,
 In deuterium,
In deuterium, ![]() , one electron (of
mass me) revolves around nucleus containing one proton and one
neutron ( of mass 2M).
, one electron (of
mass me) revolves around nucleus containing one proton and one
neutron ( of mass 2M).
![]() Reduced mass for
deuterium,
Reduced mass for
deuterium,
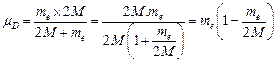 When an
electron jumps from orbit j o orbit I, the frequency of radiation emitted
When an
electron jumps from orbit j o orbit I, the frequency of radiation emitted
![]()
![]()
![]() If
If ![]() is wavelength emitted in
case of deuterium, and
is wavelength emitted in
case of deuterium, and ![]() is wavelength emitted in
case of hydrogen,
is wavelength emitted in
case of hydrogen,

![]()
![]() As
As 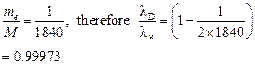
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
