Answer:
Naphthalene is insoluble in water but soluble in benzene
(organic solvent). Ammonium chloride is soluble in water but insoluble in
benzene. Naphthalene changes into vapours at room temperature whereas ammonium
chloride changes into vapours on heating.
Method
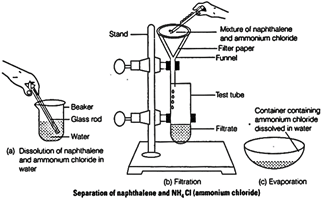
(a) Add water to the mixture and shake it vigorously to
dissolve ammonium chloride.
(b) Filter the mixture. Naphthalene is obtained as residue
whereas, filtrate contains ammonium chloride. Crystallize the filtrate by
heating till saturated solution of ammonium chloride is obtained.
(c) Cool the hot saturated solution to get crystals of ammonium chloride.
You need to login to perform this action.
You will be redirected in
3 sec
