Answer:
(d) K, L, M
Electronic configuration ![]() atom
= 2, 8, 2 and that of
atom
= 2, 8, 2 and that of ![]()
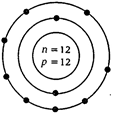 Number of protons in Mg atom=2 + 8+ 2 =12
Number of neutrons in Mg atom =24-12 = 12
as mass number of Mg atom =24 and number of neutrons .
= mass number - number of protons]
Therefore, option (d) is the correct answer.
Number of protons in Mg atom=2 + 8+ 2 =12
Number of neutrons in Mg atom =24-12 = 12
as mass number of Mg atom =24 and number of neutrons .
= mass number - number of protons]
Therefore, option (d) is the correct answer.
You need to login to perform this action.
You will be redirected in
3 sec
