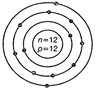
 (b)
(b) 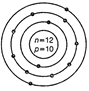 (c)
(c) 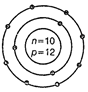 (d)
(d) 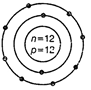 In a sample of ethyl ethanoate
In a sample of ethyl ethanoate Answer:
(c) The two O-atoms in ![]() can have
different number of neutrons only if the two 0-atoms are isotopes. It is
because, isotopes of an element have same number of protons (and electrons) but
different number of neutrons.
can have
different number of neutrons only if the two 0-atoms are isotopes. It is
because, isotopes of an element have same number of protons (and electrons) but
different number of neutrons.
You need to login to perform this action.
You will be redirected in
3 sec
