Answer:
Rutherford's model could not explain the stability of the
atom. This is because according to Rutherford's model, an atom consists of a
small heavy positively charged nucleus in the centre and the electrons were
revolving around it.
These revolving electrons would loose energy as they are
the charged particles and due to acceleration charged particles would radiate
energy.
Thus, the orbit of the revolving electron will keep on
becoming smaller and smaller, following a spiral path as shown in figure and
ultimately the electron should fall into the nucleus. In other words, the atom
should collapse.
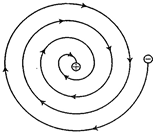 Continuous loss of energy by a revolving electron
Continuous loss of energy by a revolving electron
You need to login to perform this action.
You will be redirected in
3 sec
