Answer:
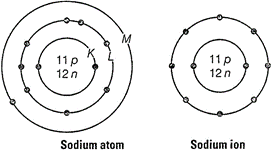
Atomic number of sodium (Z) = 11
Mass number of sodium (A) = 23
![]() Number
of protons in the nucleus = 11
Number of neutrons in the nucleus = 23 - 11 = 12
Number of electrons = 11
Number
of protons in the nucleus = 11
Number of neutrons in the nucleus = 23 - 11 = 12
Number of electrons = 11
![]() Electronic
configuration of Na atom
Electronic
configuration of Na atom ![]() Na+ ion is formed from sodium atom by loss of
an electron (present in the outermost shell).
Hence, its electronic configuration is
Na+ ion is formed from sodium atom by loss of
an electron (present in the outermost shell).
Hence, its electronic configuration is ![]() . However,
number of protons and neutrons remains the same.
. However,
number of protons and neutrons remains the same.
You need to login to perform this action.
You will be redirected in
3 sec
