Mechanism Of Absorption Of Mineral Elements
Category : 11th Class
Plants absorb the minerals from the soil and translocate them to other parts of the body. Soil serves as a main source of mineral salts in which clay crystals with a central nucleus is called micelle. The micelles are negatively charged. To maintain the balance, they hold positively charged ions on their surface. When this balance is disturbed by salt absorption, the equilibrium is again restored by transferring some of the absorbed ions into the solution. The movement of ions is called as flux. The movement of ions into the cell is called influx and outward migration of ions is known as efflux. Various theories have been proposed to explain the mechanism of mineral salt absorption and can be placed under the following two categories.
(1) Passive absorption : Absorption of ions without the use of metabolic energy is known as passive absorption. This type of absorption is carried out by purely physical forces.
In most of the cases, the movement of mineral ions into root occurs by diffusion. Diffusion of molecules is their net movement down a free energy or chemical potential gradient. The rate of diffusion varies with the chemical potential gradient or the difference in activity (essentially equivalent to concentration) across the diffusion distance.
Briggs and Robertson (1957) demonstrated the passive absorption of ions by root system. They showed :
(i) Mineral salt absorption is not affected by temperature and metabolic inhibitors.
(ii) Rapid uptake of ions occurs when plant tissues are transferred from a medium of low concentration to high concentration.
Some of the important theories explaining the mechanism of passive absorption of minerals are given below :
Mass flow hypothesis : According to Hylmo (1953, 1955), the ion absorption increases with increase in transpiration. The ions have been considered to move in a mass flow with water from the soil solution through the root and eventually to the shoot. The theory was supported by Kramer (1956), Russel and Barber (1960), etc. Later, Lopushinsky (1960) using radioactive \[{{P}^{32}}\]and \[C{{a}^{45}},\]has supported this experiment.
Simple diffusion hypothesis : According to this hypothesis, if the concentration of solutes inside the plant is lower than the soil, the mineral ions are thought to migrate into the root by simple diffusion. As a result, a state of equilibrium is reached. The part of plant cell or tissue that permits free diffusion is sometimes called outer space. The apparent volume that accomodates these ions has been referred to by some workers as apparent free space. The accumulation of ions in the cell against concentration gradient can not be explained by this concept.
Facilitated diffusion hypothesis : According to this concept, the ions are transported across the membrane by a carrier protein. When the ions enter the cell through protein channels and not through the lipid layer the phenomenon is called facilitated diffusion.
Ion exchange hypothesis : According to this view the ions adsorbed to the cell surface are exchanged from the external medium. A cation is exchanged for a cation and anion for anion. If a particular ion is absorbed by the plant, in exchange it offers \[{{H}^{+}}\]or \[O{{H}^{}}\]ions which are made available by the dissociation of water molecule.
There are two theories to explain the mechanism of ion exchange.
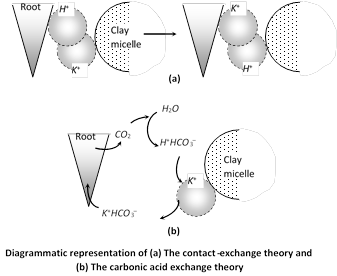
(i) Contact exchange theory : According to this theory, ions are not completely static, they are always oscillating around their absorption surface and when the oscillation volume of the ions on the roots and on the colloidal particles overlap each other, ion exchange occurs. An equilibrium is maintained between the dissolved fractions as any depletion in the soil solution is covered by movement of ions.
(ii) Carbonic acid exchange theory : In this case, \[C{{O}_{2}}\]released by roots during respiration reacts with water to produce carbonic acid which dissociates into hydrogen ions and bicarbonate ions. Hydrogen ion exchanges itself with the cations adsorbed on the colloidal particles and the bicarbonate ions release the adsorbed anions to supply both anions and cations nearby. \[{{H}_{2}}O+C{{O}_{2}}{{H}_{2}}C{{O}_{3}}{{H}^{+}}+HCO_{3}^{-}\]
Donnan equilibrium : This mechanism, given by F.G. Donnan (1927), takes into account the effect of non-diffusible ions, which may be present on one side of the membrane. Unlike diffusible ions, the membrane is not permeable to non-diffusible ions. Such ions are termed as fixed ions. They may be anions or cations. In a system, in which there are no fixed ions, there are equal number of anions and cations on both sides of the membrane at equilibrium. But in Donnan equilibrium, in order to balance the charge of the fixed ions (say anions), more ions of the other charge (say cations) would be required.
Mathematically, the Donnan equilibrium may be represented by following equation :
\[[C_{i}^{+}][A_{i}^{-}]=[C_{o}^{+}][A_{o}^{-}]\]
Here : \[C_{i}^{+}=\]Cations inside; \[C_{o}^{+}=\]Cations outside
\[A_{i}^{-}=\]Anions inside; \[A_{o}^{-}=\]Anions outside
\[\frac{\text{Positive}\,\text{ions}\,\text{inside}}{\text{Positive}\,\text{ions}\,\text{outside}}=\frac{\text{Negative}\,\text{ions}\,\text{outside}}{\text{Negative}\,\text{ions}\,\text{inside}}\]
(2) Active absorption : Generally, the lipid-protein membrane of a cell is largely permeable to free ions. The energy is considered to be involved in the transport of such free ions across the membrane. The absorption of ions, involving use of metabolic energy, is called active absorption. Energy used in these mechanisms comes from metabolic activities, especially respiration. Mineral absorption is mainly active process. Hoagland (1944) indicated active ion absorption and their (ions) accumulation against concentration gradient in green algae Nitella and Valonia.
Following evidences show the involvement of metabolic energy in the absorption of mineral salts :
(i) Higher rate of respiration increases the salt accumulation inside the cell.
(ii) Respiratory inhibitors check the process of salt uptake.
(iii) By decreasing oxygen content in the medium, the salt absorption is also decreased.
Active transport in necessary for living cells because certain substances must be concentrated and others must be excluded. Active uptake of minerals by roots mainly depends on availability of oxygen. Some of these are discussed below :
Carrier concept : This concept was proposed by Van den Honert (1937). The space in a cell or tissue where mineral ions enter by the usage of metabolic energy is called inner space. According to this concept there are separate carriers for cations and anions. A carrier forms an ion-carrier complex on the outer surface of the membrane. This complex breaks up and releases the ion into the inner space and this release is perhaps mediated by the enzyme phosphatase. The inactivated carrier is again activated by the enzyme kinase and in this process an ATP is used up. ATP molecule combine with carrier molecules and allow passage of substances against concentration gradient. The activated carrier again accepts new ions and the entire cycle is repeated.
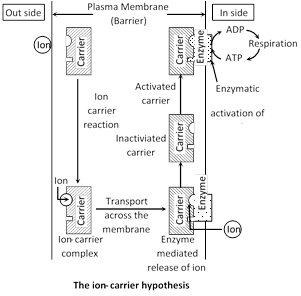
\[Carrier*+\text{ }Ion\text{ (}+/\text{)}~\xrightarrow{{}}Ion-Carrier\text{ }complex*\]
\[IonCarrier\text{ }complex*\xrightarrow{Phosphatse\,(?)}Carrier+Ion\]
\[\underset{(Inactivated)}{\mathop{Carrier}}\,+ATP\xrightarrow{Kinase}\underset{(Activated)}{\mathop{Carrier*}}\,+ADP\]
Cytochrome – pump hypothesis : This theory was proposed by Lundegardh (1950, 1954). According to this explanation only anions are absorbed actively, i.e., anion uptake requires energy and the absorption of cations does not require energy, (i.e., they are absorbed passively). At the outer surface of the membrane, the cytochrome undergoes oxidation and loses one electron and in exchange picks up an anion. This is then transported to the inner side of the membrane through to the cytochrome chain and on the inner surface of the membrane the anion is released and the cytochrome gets reduced by the action of dehydrogenase involved in respiration.
The cations move passively along the electrical gradient created by the accumulation of anions at the inner surface of the membrane.
The evidence in favour of Lundegardh's hypothesis is that the respiration increases when a plant is transferred from water to salt solution. The increased respiration is called salt respiration or anion respiration.
Criticism :
(i) It is applicable to absorption of anions only.
(ii) It fails to explain selective absorption of ions.
(iii) It has been observed that even cations can stimulate respiration.
(iv) ETS is poorly developed in anaerobically respiring forms.
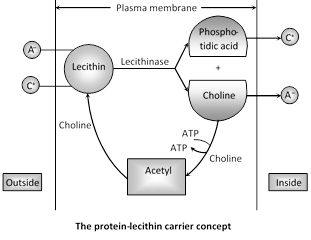
Protein-lecithin carrier concept : Bennet-Clark (1956) proposed that the carrier could be some amphoteric molecule which can carry anions as well as cations. He suggested it to be a membrane-bound protein which is conjugated with a phosphatide called as lecithin. Lecithin functions as a carrier. According to this theory, the phosphate group in the phosphatide acts as the cation binding site and choline acts as the anion binding site. During transport, ions are picked up by lecithin to form an ion-lecithin-complex. The ions are released on the inner surface of the membrane due to hydrolysis of lecithin by the enzyme lecithinase into phosphatidic acid and choline.
Lecithin is resynthesised from these components in the presence of enzyme choline acetylase and choline esterase which requires ATP.
Goldacre, 1952 proposed a mechanism of ion transport where contractile proteins act as ion carrier. They bind ions in unfolded condition on the outer face of the membrane and then contract releasing the ion into the cell and again become unfolded. The energy for this folding and unfolding is provided by ATP.
In hydrophytic plants, water and salts are absorbed by outer layer of plants.
You need to login to perform this action.
You will be redirected in
3 sec
