Hydrogen
Category : JEE Main & Advanced
(1) Position of hydrogen in the periodic table
Hydrogen is the first element in the periodic table. Hydrogen is placed in no specific group due to its property of giving electron (When \[{{H}^{-}}\] is formed) and also losing electron (When \[{{H}^{+}}\]is formed).
(i) Hydrogen is placed in group I (Alkali metals) as,
(a) It has one electron in its (Outer) shell-\[1{{s}^{1}}\] like other alkali metals which have (inert gas) \[n{{s}^{1}}\] configuration.
(b) It forms monovalent \[{{H}^{+}}\] ion like \[L{{i}^{+}},\ N{{a}^{+}}\ldots \]
(c) Its valency is also 1.
(d) Its oxide \[({{H}_{2}}O)\] is stable as \[L{{i}_{2}}O,\ N{{a}_{2}}O\].
(e) It is a good reducing agent (In atomic as well as molecular state) like \[Na\text{, }Li\ldots \]
(ii) Hydrogen also resembles halogens (Group VII A) as,
(a) It is also diatomic \[({{H}_{2}})\]like \[{{F}_{2}},\ C{{l}_{2}}\ldots \]
(b) It also forms anion \[{{H}^{-}}\] like \[{{F}^{-}},\ C{{l}^{-}}\ldots \] by gain of one electron.
(c) \[{{H}^{-}}\] has stable inert gas \[(He)\] configuration as \[C{{H}_{4}},{{C}_{2}}{{H}_{6}}\] like halogens \[CC{{l}_{4}},S{{F}_{2}}C{{l}_{2}}\] etc.
(d) H is one electron short of duplet (Stable configuration) like \[F,\ Cl,\ldots \] which are also one electron deficient than octet,\[F-2{{s}^{2}}2{{p}^{5}}\]; \[Cl-3{{s}^{2}}3{{p}^{5}}\].
(e) (IE) of \[H(1312\ kJ\ mo{{l}^{-1}})\] is of the same order as that of halogens.
(iii) (IE) of H is very high in comparison with alkali metals. Also size of \[{{H}^{+}}\] is very small compared to that of alkali metal ion. H forms stable hydride only with strongly electropositive metals due to smaller value of its electron affinity \[(72.8\,kJ\ mo{{l}^{-1}})\].
(iv) In view of the anomalous behaviour of hydrogen, it is difficult to assign any definite position to it in the periodic table. Hence it is customary to place it in group I (Along with alkali metals) as well as in group VII (Along with halogens).
(2) Discovery and occurrence : It was discovered by Henry Cavendish in 1766. Its name hydrogen was proposed by Lavoisier. Hydrogen is the 9th most abundant element in the earth's crust.
Hydrogen exists in diatomic state but in triatomicstate it is called as Hyzone. Systematic name of water is oxidane.
(3) Preparation of Dihydrogen : Dihydrogen can be prepared by the following methods,
(i) By action of water with metals
(a) Active metals like Na, K react at room temperature
\[2M+2{{H}_{2}}O\to 2MOH+{{H}_{2}}\] [M = Na, K etc.]
(b) Less active metals like Ca, Zn, Mg, Al liberate hydrogen only on heating.
\[2Al+3{{H}_{2}}O\to A{{l}_{2}}{{O}_{3}}+3{{H}_{2}}\]
(c) Metals like Fe, Ni, Co, Sn can react only when steam is passed over red hot metals.
\[3Fe+4{{H}_{2}}O(\text{steam})\to \underset{\text{Ferrosoferric oxide}}{\mathop{F{{e}_{3}}{{O}_{4}}}}\,+4{{H}_{2}}\]
(ii) By the action of water on alkali and alkaline earth metals hydrides
\[NaH+{{H}_{2}}O\to NaOH+{{H}_{2}}\]
\[Ca{{H}_{2}}+2{{H}_{2}}O\to Ca{{(OH)}_{2}}+2{{H}_{2}}\]
(iii) By reaction of metals like Zn, Sn, Al with alkalies (NaOH or KOH)
\[Zn+2NaOH\xrightarrow{\Delta }\underset{\text{sod}\text{. zincate}}{\mathop{N{{a}_{2}}Zn{{O}_{2}}}}\,+{{H}_{2}}\]
\[Al+2NaOH+{{H}_{2}}O\xrightarrow{\Delta }\underset{\text{Sod}\text{. meta-aluminate}}{\mathop{2NaAl{{O}_{2}}}}\,+2{{H}_{2}}\]
\[\underset{\text{Silicon}}{\mathop{Si}}\,+2NaOH+2{{H}_{2}}O\xrightarrow{\Delta }N{{a}_{2}}Si{{O}_{3}}+3{{H}_{2}}\]
\[\underset{\text{Tin}}{\mathop{Sn}}\,+2NaOH\xrightarrow{\Delta }\underset{\text{Sod}\text{. stannite}}{\mathop{N{{a}_{2}}Sn{{O}_{2}}}}\,+{{H}_{2}}\uparrow \]
(iv) By action of metal with acids : All active metals which lie above hydrogen in electrochemical series, can displace hydrogen gas from dilute mineral acids like HCl, \[{{H}_{2}}S{{O}_{4}}\].
\[Fe+2HCl\to FeC{{l}_{2}}+{{H}_{2}}\]
(v) By the electrolysis of acidified water
\[2{{H}_{2}}O\xrightarrow{{{H}^{+}}/\text{Electrolysis}}\underset{\text{At cathode}}{\mathop{2{{H}_{2}}\uparrow }}\,+\underset{\text{At anode}}{\mathop{{{O}_{2}}\uparrow }}\,\]
(vi) Laboratory method : In laboratory, it is obtained by action of granulated zinc with dilute \[{{H}_{2}}S{{O}_{4}}\].
\[Zn+\text{dil}\text{. }{{H}_{2}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}\]
It must be noted that
(a) Pure zinc is not used for the preparation of \[{{H}_{2}}\] as rate of reaction of pure Zn with dil.\[{{H}_{2}}S{{O}_{4}}\] is quite slow.
(b) Conc.\[{{H}_{2}}S{{O}_{4}}\] is not used because then \[S{{O}_{2}}\] gas is evolved instead of \[{{H}_{2}}\].
(vii) Preparation of pure hydrogen: It can be obtained by (a) The action of pure dil.\[{{H}_{2}}S{{O}_{4}}\] on pure magnesium ribbon.
\[Mg+{{H}_{2}}S{{O}_{4}}\to MgS{{O}_{4}}+{{H}_{2}}\]
(b) Hydrogen of high purity (> 99.95%) is obtained by electrolysing warm aqueous barium hydroxide between nickel electrodes.
(c) By the action of water on sodium hydride.
\[NaH+{{H}_{2}}O\to NaOH+{{H}_{2}}\uparrow \]
(d) By the action of KOH (aq.) on aluminium.
\[2Al+2KOH+2{{H}_{2}}O\to 2KAl{{O}_{2}}+3{{H}_{2}}\uparrow \]
(viii) Commercial production of dihydrogen
(a) Bosch process : In this method, water gas is mixed with twice its volume of steam and passed over heated catalyst \[F{{e}_{2}}{{O}_{3}}\] in the presence of a promoter \[C{{r}_{2}}{{O}_{3}}\] or \[Th{{O}_{2}}\] at 773 K when \[C{{O}_{2}}\] and \[{{H}_{2}}\] are obtained. \[C{{O}_{2}}\] is removed by dissolving it in water under pressure (20-25 atm) and \[{{H}_{2}}\] left undissolved is collected.
\[C+{{H}_{2}}O\xrightarrow{1270\,K}\underbrace{CO+{{H}_{2}}}_{\text{Water gas}}\]
\[{{H}_{2}}+CO+{{H}_{2}}O\underset{F{{e}_{2}}{{O}_{3}},\,C{{r}_{2}}{{O}_{3}}}{\mathop{\xrightarrow{773\,K}}}\,C{{O}_{2}}+2{{H}_{2}}\]
About 18% of the world?s production of \[{{H}_{2}}\] is obtained from coal.
(b) Lane's process : By passing steam over spongy iron at 773-1050 K.
\[3Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\]
The ferrosoferric oxide \[(F{{e}_{3}}{{O}_{4}})\] so produced is reduced back to iron with water. this reaction is known as Vivification reactions
\[F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\to 3Fe+4{{H}_{2}}O\]
\[F{{e}_{3}}{{O}_{4}}+4CO\to 3Fe+4C{{O}_{2}}\]
(c) By electrolysis of water : Electrolysis of acidified water using platinum electrodes is used for the bulk preparation of hydrogen.
(d) From hydrocarbons : Hydrocarbons (alkanes) react with steam at high temperature to produce carbon monoxide and hydrogen, e.g.,
\[C{{H}_{4}}(g)+{{H}_{2}}O(g)\underset{\text{Catalyst}}{\mathop{\xrightarrow{1270\,K}}}\,CO(g)+3{{H}_{2}}(g)\]
The mixture of CO and \[{{H}_{2}}\] so obtained can be converted into hydrogen as in Bosch process. About 77% of the world?s production of \[{{H}_{2}}\] is obtained from hydrocarbons.
(e) It is also produced as a by-product of the brine electrolysis process for the manufacture of \[C{{l}_{2}}\] and NaOH.
(4) Physical properties of dihydrogen : It is a colourless, tasteless and odourless gas. It is slightly soluble in water. It is highly combustible. The Physical constants of atomic hydrogen are,
Atomic radius (pm) - 37
Ionic radius of \[{{H}^{-}}\] ion (pm) - 210
Ionisation energy \[(kJ\,mo{{l}^{-1}})\] - 1312
Electron affinity \[(kJ\,mo{{l}^{-1}})\] - 72.8
Electronegativity - 2.1
(5) Chemical properties of dihydrogen : Dihydrogen is quite stable and dissociates into hydrogen atoms only when heated above 2000 K, \[{{H}_{2}}\xrightarrow{2000\,K}H+H\]. Its bond dissociation energy is very high, \[{{H}_{2}}\to H+H\,;\ \ \,\Delta H=435.9\,kJ\,mo{{l}^{-1}}\]. Due to its high bond dissociation energy, it is not very reactive. However, it combines with many elements or compounds.
(i) Action with metals : To forms corresponding hydrides. \[2Na+{{H}_{2}}\xrightarrow{Heat}2NaH\]; \[Ca+{{H}_{2}}\xrightarrow{Heat}Ca{{H}_{2}}\].
With transition metals (elements of d ? block) such as Pd, Ni, Pt etc. dihydrogen forms interstitial hydrides in which the small molecules of dihydrogen occupy the interstitial sites in the crystal lattices of these hydrides. As a result of formation of interstitial hydrides, these metals adsorb large volume of hydrogen on their surface. This property of adsorption of a gas by a metal is called occlusion. The occluded hydrogen can be liberated from the metals by strong heating.
(ii) Reaction with Non-metals
\[2{{H}_{2}}+{{O}_{2}}\xrightarrow{970\,K}2{{H}_{2}}O\]
\[{{N}_{2}}+3{{H}_{2}}\overset{Fe,\,Mo}{\mathop{\xrightarrow[750\,K,\,\text{Pressure}]{}}}\,2N{{H}_{3}}\]
\[{{H}_{2}}+{{F}_{2}}\xrightarrow{Dark}2HF\]
\[{{H}_{2}}+C{{l}_{2}}\overset{Sunlight}{\mathop{\xrightarrow[673\,K,\,\text{Pressure}]{}}}\,2HCl\]
\[{{H}_{2}}+B{{r}_{2}}\to 2HBr\]
\[{{H}_{2}}+{{I}_{2}}\underset{Pt}{\mathop{\xrightarrow{673\,K}}}\,2HI\]
The reactivity of halogen towards dihydrogen decreases as, \[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{I}_{2}}\]
As a result, \[{{F}_{2}}\]reacts in dark, \[C{{l}_{2}}\] in the presence of sunlight, \[B{{r}_{2}}\] reacts only upon heating while the reaction with \[{{I}_{2}}\] occurs in the presence of a catalyst.
(iii) Reaction with unsaturated hydrocarbons : \[{{H}_{2}}\] reacts with unsaturated hydrocarbons such as ethylene and acetylene to give saturated hydrocarbons.
\[\underset{Ethylene}{\mathop{{{H}_{2}}C=C{{H}_{2}}}}\,+{{H}_{2}}\underset{473\,K}{\mathop{\xrightarrow{Ni\,or\,Pt\,or\,Pd}}}\,\underset{Ethane}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\]
\[\underset{Acetylene}{\mathop{HC\equiv CH}}\,+2{{H}_{2}}\underset{473\,K}{\mathop{\xrightarrow{Ni\,or\,Pt\,or\,Pd}}}\,\underset{Ethane}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\]
This reaction is used in the hydrogenation or hardening of oils. The vegetable oils such as groundnut oil or cotton-seed oil are unsaturated in nature because they contain at least one double bond in their molecules. Dihydrogen is passed through the oils at about 473 K in the presence of catalyst to form solid fats. The vegetable ghee such as Dalda, Rath, etc. are usually prepared by this process.
\[\underset{(liquid)}{\mathop{\text{Vegetable}\,\text{oil}}}\,+{{H}_{2}}\overset{Ni}{\mathop{\xrightarrow[473\,K]{}}}\,\underset{(solid)}{\mathop{Fat}}\,\]
(6) Uses of Dihydrogen
(i) As a reducing agent
(ii) In the hydrogenation of vegetable oils
(iii) As a rocket fuel in the form of liquid \[{{H}_{2}}\]
(iv) In the manufacture of synthetic petrol
(v) In the preparation of many compounds such as \[N{{H}_{3}},\,C{{H}_{3}}OH,\,\]Urea etc.
(vi) It is used in the oxy-hydrogen torch for welding if temperature around 2500°C is required. It is also used in atomic hydrogen torch for welding purposes in which temperature of the order of 4000°C is required.
Different forms of hydrogen
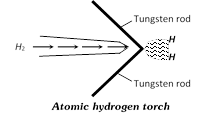
(1) Atomic hydrogen : It is obtained by the dissociation of hydrogen molecules.
The atomic hydrogen is stable only for a fraction of a second and is extremely reactive. It is obtained by passing dihydrogen gas at atmospheric pressure through an electric arc struck between two tungsten rods. The electric arc maintains a temperature around 4000 - 4500°C.
As the molecules of dihydrogen gas pass through the electric arc, these absorb energy and get dissociated into atoms as
\[{{H}_{2}}(g)\underset{arc}{\mathop{\xrightarrow{Electric}}}\,2H(g):\Delta H=435.90KJ\,mo{{l}^{-1}}\]
This arrangement is also called atomic hydrogen torch.
(2) Nascent hydrogen : The hydrogen gas prepared in the reaction mixture in contact with the substance with which it has to react, is called nascent hydrogen. It is also called newly born hydrogen. It is more reactive than ordinary hydrogen. For example, if ordinary hydrogen is passed through acidified \[KMn{{O}_{4}}\] (pink in colour), its colour is not discharged. On the other hand, if zinc pieces are added to the same solution, bubbles of hydrogen rise through the solution and the colour is discharged due to the reduction on \[KMn{{O}_{4}}\] by nascent hydrogen.
\[KMn{{O}_{4}}+\underset{Molecular}{\mathop{{{H}_{2}}}}\,+{{H}_{2}}S{{O}_{4}}\to No\,reaction\]
\[Zn+{{H}_{2}}S{{O}_{4}}\to ZnS{{O}_{4}}+\underset{Nascent\,hydrogen}{\mathop{2[H]}}\,\]
\[2KMn{{O}_{4}}+3{{H}_{2}}S{{O}_{4}}+10[H]\to {{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+8{{H}_{2}}O\]
(3) Ortho and para hydrogen : A molecule of dihydrogen contains two atoms. The nuclei of both the atoms in each molecule of dihydrogen are spinning. Depending upon the direction of the spin of the nuclei, the hydrogen is of two types,

(i) Molecules of hydrogen in which the spins of both the nuclei are in the same directions, called ortho hydrogen.
(ii) Molecules of hydrogen in which the spins of both the nuclei are in the opposite directions, called para hydrogen.
Ordinary dihydrogen is an equilibrium mixture of ortho and para hydrogen. Ortho hydrogen ? Para hydrogen. The amount of ortho and para hydrogen varies with temperature as,
(a) At 0°K, hydrogen contains mainly para hydrogen which is more stable.
(b) At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is 1:1.
(c) At the room temperature, the ratio of ortho to para hydrogen is 3:1.
(d) Even at very high temperatures, the ratio of ortho to para hydrogen can never be more than 3:1.
Thus, it has been possible to get pure para hydrogen by cooling ordinary hydrogen gas to a very low temperature (close to 20 K) but it is never possible to get a sample of hydrogen containing more than 75% of ortho hydrogen. i.e., Pure ortho hydrogen can not be obtained.
(4) Hydrides : Hydrogen forms binary hydrides of the type \[M{{H}_{x}}\] or \[{{M}_{m}}{{H}_{n}}\] with
(a) All main group elements except noble gases and probably indium and thallium.
(b) All lanthanoids and actinoids.
(c) Transition metals (Sc, Y, La, Ac, Tc, Zr, Hf and to a lesser extent V, Nb, Ta, Cr, Cu and Zn). In group 6 only Cr forms hydride (CrH).
Hydrides are classified into three main categories.
(i) Saline or ionic hydrides : Most of the s-block metals form this type of hydrides. These are non-volatile, non-conducting crystalline solids. However, \[Be{{H}_{2}}\] and \[Mg{{H}_{2}}\] have covalent polymeric structure. These ionic hydrides have rock-salt structure. Thermal stability of 1st and 2nd group hydrides are in the order;
LiH > NaH > KH > RbH > CsH
\[Ca{{H}_{2}}>Sr{{H}_{2}}>Ba{{H}_{2}}\]
\[Be{{H}_{2}},\ Mg{{H}_{2}}\] and LiH have significant covalent character.
Electrolysis of solution of saline hydride in molten alkali halide produces \[{{H}_{2}}\] at anode. Saline hydrides react explosively with water.
\[NaH(s)+{{H}_{2}}O(aq)\to NaOH(aq)+{{H}_{2}}(g)\]
The fire so produced cannot be extinguished by \[C{{O}_{2}}\] as it gets reduced by the hot metal hydride. Only sand is useful, as it is a solid.
Alkali metal hydrides are used for making \[LiAl{{H}_{4}},\ NaB{{H}_{4}}\] etc. Alkali metal hydrides are also used for the removal of last traces of water from organic compounds.
(ii) Metallic or interstitial hydrides : Elements of groups 3, 4, 5 (d-block) and f-block elements form metallic hydrides. In group 6, only Cr forms hydride (CrH). Metals of group 7, 8, 9 do not form hydrides. This region of periodic table from group 7 to group 9 is known as hydride gap. Examples of hydrides of group 3 to 5 are, \[Sc{{H}_{2}},\,Y{{H}_{2}},\,Y{{H}_{3}},\,La{{H}_{2}},\,La{{H}_{3}},\,Ti{{H}_{2}},\,Zr{{H}_{2}},\,Hf{{H}_{2}},\,VH,\]
\[V{{H}_{2}},\,NbH,\,Nb{{H}_{2}},\,TaH\]
The f-block metals form hydrides of limiting compositions of \[M{{H}_{2}}\] and \[M{{H}_{3}}\]. All these hydrides are non-stoichiometric with variable composition e.g.,
\[Zr{{H}_{x}}(1.30\le x\le 1.75)\], \[Ti{{H}_{x}}(1.8\le x\le 2.0)\].
Most of these hydrides are good conductors of electricity in solid state.
Metallic hydrides can be used to store hydrogen especially in cars working on fuel cells.
(iii) Molecular or covalent hydrides : Hydrogen form molecular compounds with p-block elements (B, C, N, O, F; Si, P, S, Cl; Ga, Ge, As, Sb, Br; In, Sn, Sb, Te, I; Tl, Pb, At). common examples of such hydrides are \[C{{H}_{4}},\,N{{H}_{3}},\,{{H}_{2}}O,\,HF\]etc. The stability of these hydrides decreases down the group. For example, \[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}>Bi{{H}_{3}}\]. In a period the stability increases with increasing electronegativity. For example, \[C{{H}_{4}}<N{{H}_{3}}<{{H}_{2}}O<HF\]. Molecular hydrides are classified as electron rich, electron precise and electron deficient hydrides.
(a) Electron rich molecular hydrides : These hydrides have one or more lone pairs of electrons around the central more electronegative element. For example
\[H-\underset{.\ .}{\overset{\cdot \ \cdot }{\mathop{O}}}\,-H\], \[H-\underset{H}{\mathop{\underset{|}{\overset{\cdot \ \cdot }{\mathop{N}}}\,}}\,-H\], \[H-\underset{.\ .}{\overset{\cdot \ \cdot }{\mathop{F}}}\,:\]
(b) Electron precise molecular hydrides : Elements of group 14 form such hydrides. The bond length increases on going down the group. A common example of electron precise molecular hydrides is \[C{{H}_{4}}\].
(c) Electron deficient molecular hydrides : These hydrides have lesser number of electrons than that required for writing the conventional Lewis structure. A common example of such molecular hydride is diborane, \[{{B}_{2}}{{H}_{6}}\].
(d) Systematic names of molecular hydrides : The systematic names of these hydrides are obtained from the name of the element and the suffix ?ane. For example,
\[\underset{\text{Phosphane}}{\mathop{P{{H}_{3}}}}\,\] \[\underset{\text{oxidane}}{\mathop{{{H}_{2}}O}}\,\] \[\underset{\text{ozane}}{\mathop{N{{H}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
