Chemical Reaction
Category : 7th Class
The chemical reaction is the process in which two substances combine and form a new substance. Chemical reactions are widely used for the formation of desired substances. The substances, which are initially involved in the chemical reaction are called reactants. And the substance, which we get after completion of chemical reaction, is known as products.
Look at the following chemical reaction of Hydrogen and Oxygen
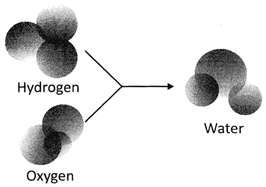
In the above picture, molecules of hydrogen and oxygen combine together to form molecules of water.
![]()
![]() Types of Reactions
Types of Reactions
Types of reaction depend on nature of the combination of reactants and energy released or absorbed by the resulting chemical, after the reaction. There are three types of reaction, which have been discussed below:
![]() Direct Combination or Synthesis Reaction
Direct Combination or Synthesis Reaction
In the synthesis reaction, two molecules combine chemically to form a more complex molecule and energy of the resulting molecule is stored in its bonds. Proteins are built from amino acid and whole energy is stored in it.
Example: Iron and sulfur combine to form iron sulfide.
![]()
![]() Decomposition Reactions
Decomposition Reactions
In this reaction, the bigger molecule is broken into smaller molecule and energy is released.
The electrolysis of water into hydrogen and oxygen is the decomposition or analysis reaction.
![]()
![]() Exchange Reaction
Exchange Reaction
In this reaction, reactants exchange and replace their parts to form new compounds. When sodium chloride and silver nitrate are mixed together, sodium nitrate and silver chloride are formed.
![]()
The combination of zinc and hydrochloric acid forms zinc chloride and it replaces the hydrogen..
![]()
![]() Acid and Base Reaction
Acid and Base Reaction
When in a chemical reaction, acid and base are involved as a reactants, the reaction is called acid and base reaction.
The reaction between hydrochloric acid and sodium hydroxide produces sodium chloride and water. It is an example of acid base reaction. The chemical equation is as follows,
![]()

![]() The word reactant is derived from which one of the following process, in which a new substance is formed?
The word reactant is derived from which one of the following process, in which a new substance is formed?
(a) Mathematical process
(b) Hydraulic process
(c) Chemical process
(d) All of these
(e) None of these
Answer: (c)
![]() Which one of the following types of reaction is involved for the formation of iron sulfide from iron and Sulphur?
Which one of the following types of reaction is involved for the formation of iron sulfide from iron and Sulphur?
(a) Decomposition reaction
(b) Acid base reaction
(c) Synthesis reaction
(d) All of these
(e) None of these
Answer: (c)
You need to login to perform this action.
You will be redirected in
3 sec
