Heat
Category : 7th Class
Learning Objectives
Introduction
We wear woolen clothes during winters when it is cold outside. Woolen clothes keep us warm. In summers we feel hot inside the house as well. How do we know whether an object is hot or cold? We find some objects are hotter than others while some are colder than others. We often decide it by touching the objects, in the case of a hot body, heat flows into our body while in case of a cold body heat flows out of our body.
HEAT AND TEMPERATURE
Heat is a form of energy which causes sensation of hotness and coldness. Heat always flows from the hot object to the cold object. Temperature is the degree of hotness or coldness of. a body. Temperature determines the direction of flow of heat. Heat energy flows from a body at higher temperature to the body at lower temperature. The transfer of heat continues till the temperature of both the bodies become same. i.e., the bodies are said to be in equilibrium. Heat is a cause while temperature is an effect. The S.I. unit of heat is joule (j) because heat is a form of energy. Conventionally, heat is measured in calories (Cal). The amount of heat energy required to raise the temperature of 1 g of water by 1°C is called 1 calorie.
\[\text{1calorie}=\text{4}.\text{2 joule}\]
Do you know?
\[100c{{m}^{3}}\] of water and the \[10c{{m}^{3}}\]of water are equally hot. But \[10c{{m}^{3}}\]of water contains \[1/10\]heat than the \[100c{{m}^{3}}\] of water.
TEMPERATURE MEASURING SCALES
Temperature is measured by an instrument called thermometer.
The three scales of temperature are
(i) Celsius scale
(ii) Fahrenheit scale and
(iii) Kelvin scale
(i) Celsius Scale: Introduced by Swedish astronomer Celsius. It has 100 divisions, where each division measures one degree celsius (°C). The lower fixed point is marked as 0°C which gives the freezing point of water and upper fixed point is marked as 100°C and measures the boiling point of water.
(ii) Fahrenheit Scale: This scale was introduced by Fahrenheit. This scale has 180 divisions with each division measuring 1°F on this scale. 32°F gives the melting point of ice and 212°F gives the steam point. Fahrenheit scale is used for meterological and clinical purposes.
(iii) Kelvin Scale: It is known as absolute scale of temperature. It was introduced by Lord Kelvin. The lowest limit of temperature is absolute zero. Zero kelvin corresponds to -273°C.
Conversion of Temperature Scales
\[\frac{C}{5}=\frac{F-32}{9}\]
And, value in °C + 273 = value in Kelvin
Do you know?
When two substances are mixed together, the final temperature of the mixture can never be less than the temperature of the colder substance or more than the temperature of the hotter substance.
TEMPERATURE MEASURING INSTRUMENT-THERMOMETERS
Two types of thermometers are there in use. These are, laboratory thermometer and clinical thermometer.
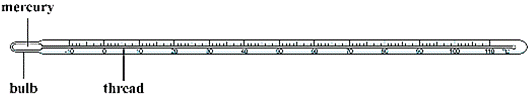
Clinical thermometer is used for the measurement of body temperature. The body temperature varies slightly. Therefore the range of clinical thermometer is between 35°C to 42°C. Clinical thermometer has graduated scale, on which scale is mentioned in degree Celsius and in Fahrenheit. Small division on the Body of the clinical thermometer represents the temperate of 0.1 °C or — degree C. The range in terms of Fahrenheit on the body of the thermometer is between 94°C to 108°C. Human body temperature on the Fahrenheit scale is 98.4°F and in the Celsius scale it is 37°C. Clinical thermometer is made up of glass tube and a liquid (mercury or alcohol or hydrocarbon based fluid) is filled inside it. As the temperature increases, volume of the mercury expands. Division on the body of the thermometer shows the increase in temperature.
Do you know?
Galileo was the first scientist to contract a thermometer base on the thermal expansion and he called his thermometer the thermo scope
Digital thermometers are widely used because of their accuracy. A digital display on it shows temperature. It requires battery for function.
Laboratory thermometer is generally available in the range of-10°C to 110°C. The graduated laboratory thermometer is used in the laboratory for the measurement of the temperature of chemicals and for other purposes.
HEAT TRANSFER
Since heat is a form of energy, it can be transferred from one part of a body to another part or from one body to another body. This transfer of heat depends upon the difference of temperatures between the two bodies.
The transfer of heat can take place by the following three modes:
(1) Conduction
(2) Convection
(3) Radiation
Do you know?
Mercury though a liquid is heated by conduction and not by convection.
(1) Conduction: It is a mode of heat transfer from particle to particle without any actual movement of the particles.
E.g., if we heat one end of a metal rod the other end of the rod also becomes hot. Here the heat has travelled from the hot end to the cold end by the process of conduction. All solids generally get heated by conduction. Touching a hot object and being burnt, cooling down of your hand on holding ice are also examples of conduction of heat.
(2) Convection: It is a mode of heat transfer from one part of a medium to another part by the actual movement of the heated particles of the medium.
Generally all fluids (liquids and gases) get heated up by convection. The phenomena of sea breeze during the day and land breeze during the night are based on natural convection.
(i) Sea breeze: During the day, the heat from the sun reaches the land and sea water on earth. But land gets heated up faster than the sea water, so the air in contact with the land is heated up and becomes lighter. As a result it rises up. The cooler air from above the sea moves down to fill the space. This creates a sea breeze.
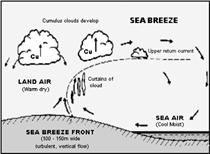
Do you know?
If liquids and gases are heated form the top they transfer heat by conduction not by convection.
(ii) Land breeze: At night the land loses its heat faster than the sea water. So the air above the sea water is warmer and it rises up. Therefore the cooler air above the land blows towards the sea and fill the space. As a result, the land breeze is set up.

(3) Radiation: Radiation is a mode of heat transfer from the source to the receiver without any actual movement of the source or the receiver and also without heating the intervening medium. Transfer of heat through radiation does not require any medium.
We feel warm when we receive heat from the sun. This heat is transferred to us by the mode of radiation. Heat from fire is also an example of radiation.
The greenhouse works on radiation. Heating up of a room through an electric heater happens because of radiation.
Do you know?
Heat radiation travels with a velocity of light \[i.e.3\times {{10}^{8}}m/s\]
CONDUCTORS AND INSULATORS
Materials that allow heat energy to pass through them easily are called conductors. Metals are good conductors of heat.
Applications of Conductors
Materials that do not allow heat energy to pass through them easily are called insulators. Substances like glass, wood, plastic, rubber and non-metals are generally insulators.
Do you know?
Silver is the best conductor of heat followed by copper and aluminum
Applications of Insulators
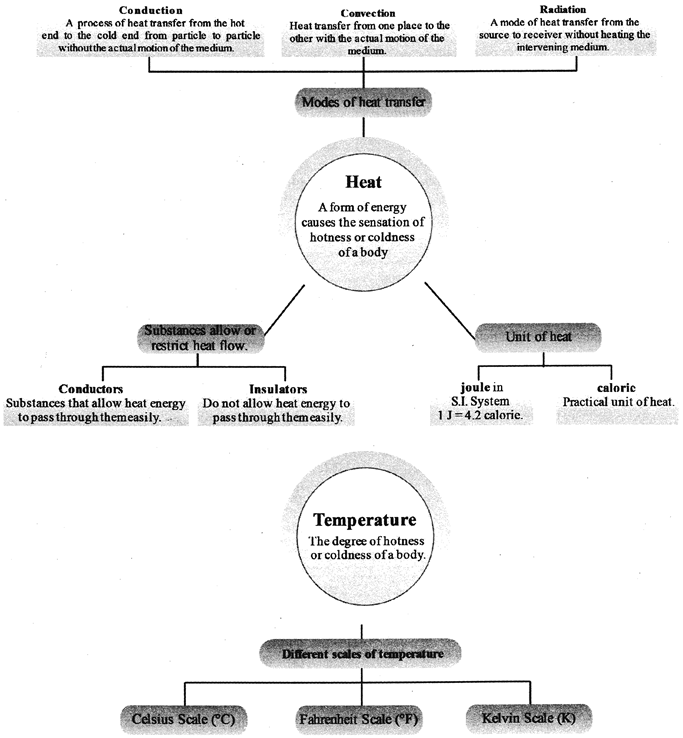
CONCEPT MAP
You need to login to perform this action.
You will be redirected in
3 sec
