Physical and Chemical changes
Category : 7th Class
Learning Objectives
We have learnt in previous class about different changes around us like periodic and non-periodic, slow and fast changes, reversible and irreversible changes around us and their causes. Every change has a cause for example days change tonight because of rotation of earth around the sun, melting of ice is due to heat supplied. There are changes in everything. When we eat, breathe, talk or sleep changes take place in our body Changes can be in shape, size, temperature, state of substance etc. In this section we will learn about the classification of the changes around us into two main categories- Physical and chemical changes.

PHYSICAL PROPERTIES
The properties which describe the look or feel of a substance e.g., colour, hardness, density, texture and phase of matter, etc. Every substance has its own characteristic physical properties that we use to identify the substance.
PHYSICAL CHANGES
A change in which physical properties of substance such as, size, appearance or state, may alter, but its chemical composition remains the same and no new substance is formed are called physical changes. A physical change is a reversible change. For example dissolving sugar in water, cutting of wood etc.
Characteristics of Physical Changes
Note:- By using a piece of gold, a goldsmith can make a large variety of ornaments. But all of them consist of the same substance, namely gold.
Some Other Examples of Physical Changes
1. Change of state- On heating water evaporates and ice melts, but on cooling water vapours changes to water and water freezes to form ice. Therefore change in state of water is a physical change.

Note: A mixture consist of two or more substances simply mixed together but not chemically combined. For example air is a mixture of various gases.
Do you know?
Component of a mixture can be separated by different separation techniques like hand picking, sedimentation, decantation, evaporation, condensation etc.
CHEMICAL CHANGES
A change in which the identity of the original substance is changed and a new substance or new substances are formed is called a chemical change. It is irreversible change.
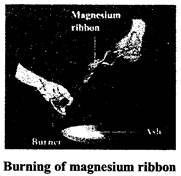
In chemical change the properties of the substance gets changed e.g., souring of milk, burning of paper, burning of candle, rusting of iron, burning of magnesium ribbon. There are various chemical changes taking place in our body for example digestion of food .water we drink is used and urine if formed, air we breathe is used in respiration, etc.
Do you know?
It may be interesting to note that tearing of paper is physical change but burning of paper is a chemical change. Changing of water to steam or ice is a physical change but formation of hydrogen and oxygen gases by passing electric current is a chemical change.
Characteristics of Chemical Changes
5. A chemical change is also a chemical reaction. A chemical reaction may also accompanied by change in colour, production of sound, change in smell or formation of gas.

Note- A compound is a substance that has been formed when two or more elements combine chemically.
Note:- In a chemical reaction the original substance is called reactant and new substance formed is called product.
Some Examples of Chemical Changes
When magnesium ribbon is burnt, it bums with a brilliant white light and when completely burnt it leaves behind a powdery ash. The chemical equation for this chemical change is
Magnesium (Mg) + Oxygen (O) \[\xrightarrow{{}}\]Magnesium oxide (MgO) (Powdery ash)
The powdery ash is different from magnesium. It is magnesium oxide (basic substance).
When magnesium oxide is dissolved in water it forms magnesium hydroxide.
Magnesium oxide \[\left( \text{MgO} \right)\] + Water (\[{{\text{H}}_{\text{2}}}\text{O}\]) \[\xrightarrow{{}}\]Magnesium hydroxide [\[\text{Mg(OH}{{\text{)}}_{\text{2}}}\]].
Magnesium hydroxide is basic in nature.
Blue vitriol is copper sulphate. It dissolves in water completely if we add a few drops of dilute sulphuric acid to it. The colour of solution so obtained is blue.
Now, if we drop an iron nail to this blue solution and allow it to stand for 30 minutes. The blue colour changes to green. The green colour is due to formation of iron sulphate due to reaction between copper sulphate (blue) and iron.
\[\underset{(\text{blue})}{\overset{{}}{\mathop{\text{Copper sulphate solution}}}}\,+\text{Iron}\xrightarrow{\text{ }}\]
\[\underset{(\text{green})}{\overset{{}}{\mathop{\text{Iron sulphate solution}}}}\,+\underset{\begin{smallmatrix}
\text{(brown} \\
\text{deposit)}
\end{smallmatrix}}{\overset{{}}{\mathop{\text{Copper}}}}\,\]
\[\underset{(\text{acetic acid})}{\overset{{}}{\mathop{\text{Vinegar}}}}\,\text{+}\underset{\begin{smallmatrix}
(\text{sodium hydrogen} \\
\text{carbonte})
\end{smallmatrix}}{\overset{{}}{\mathop{\text{Baking soda}}}}\,\xrightarrow{{}}~\underset{\text{(gas}\,\text{comes}\,\text{out)}}{\overset{{}}{\mathop{\text{Carbon dioxide}}}}\,+\text{Other substances}.\]
\[\underset{(\text{C}{{\text{O}}_{\text{2}}})}{\overset{{}}{\mathop{\text{Carbon}}}}\,\text{ dioxide+}\underset{[\text{Ca(OH}{{\text{)}}_{\text{2}}}]}{\overset{{}}{\mathop{\text{Lime water}}}}\,\xrightarrow{{}}\underset{(\text{CaC}{{\text{O}}_{\text{3}}})}{\overset{{}}{\mathop{\text{Calcium carbonate}}}}\,\text{+Water(}{{\text{H}}_{\text{2}}}\text{O)}\][The lime water turns milky due to formation of calcium carbonate]

Do you know?
Browning of food may cause fruits and vegetables to lose their appeal but there is no loss of their nutritive value. Certain substances present in some of the fruits and vegetables have a great affinity (bond) for taking up oxygen from the air. The oxygen reacts with these substances turning them into dark brown enzyme called “tyrosine’s” which is responsible for the darkening of the cut surfaces of potatoes, brinjals and apples.
RUSTING OF IRON
When a piece of iron is left in open for some time, it acquires a film of brownish substance which is called rust. This process of change of iron to rust is called rusting of iron. Rust is different from iron. This rust is iron oxide. The chemical equation for rusting of iron can be represented as:
\[\text{Iron(Fe)+Oxygen(}{{\text{O}}_{\text{2}}})+Water({{H}_{2}}O)\]
\[\xrightarrow{{}}\text{Rust(iron oxide, F}{{\text{e}}_{\text{2}}}{{\text{O}}_{3}})\]
Do you know?
It is estimated that about 15% of the total world production of iron is destroyed due to rusting.
Do you know?
Near the Qutub Minar in Delhi stands a 7 meter high iron pillar braving heat, rain and rough weather for more than 1600 years. It has not rusted even after so many years. Its quality of rust resistant iron is still a mystery for the present day scientists.
Prevention of Rusting
(i) Galvanization: It is considered as the best method to prevent rusting. It is the process of deposition of a thin layer of zinc on iron surface. It is done to prevent rusting of iron. Rusting can also be prevented by deposition of thin layer of chromium on iron articles. We use Galvanized Iron (G I.) pipes to carry water in taps.
(ii) Application of coat of paint or grease on the surface of iron also help in prevention of rusting.
Essential Conditions for Rusting
(i) Presence of moisture (water vapour)
(ii) Presence of air (or oxygen)
Factors that help in speeding up the process of rusting
(i) Higher moisture content in air increases the rate of rusting.
(ii) Presence of salts (electrolytes) in water also makes the process of rusting faster. Due to presence of many salts in sea water, the ships suffer a lot of damage from rusting in spite of being painted.


CRYSTALLISATION
When a hot saturated solution of an impure compound is allowed to cool slowly undisturbed in a beaker or in a crystallizing dish, after some time the crystals of pure compound are formed and impurities are left behind in solution.
Note: Crystallization is an example of physical change, in it no new substance is formed.
Crystallization is a process of obtaining pure compound from impure compound i.e., it is a method of purification.
For Example:
1. This process is used to get crystals of pure copper sulphate from impure sample of copper sulphate.
2. Table salt is obtained from sea water by crystallization process.
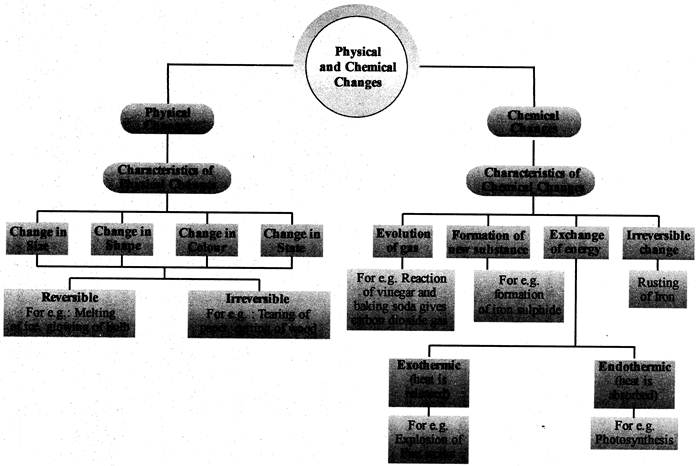
CONCEPT MAP
You need to login to perform this action.
You will be redirected in
3 sec
