Atoms and Molecules
Category : 9th Class
Atoms and Molecules
All the matters found in this universe are made up of small particles called atoms. Atoms combine to form molecule. Different atoms and molecules have different properties due to which different matters show different properties.
Laius of Chemical Combination
The laws of chemical combination are:
Law of Conservation of Mass
This law states that in a chemical reaction, the total mass of products is equal to the total mass of reactants. There occurs no change in mass during a chemical reaction. For example, in the following chemical reaction:
Sodium carbonate + ethanoic acid \[\to \] sodium ethanoate + carbon dioxide + water
The addition of mass of sodium carbonate and ethanoic acid will be equal to the addition of mass of sodium ethanoate, carbon dioxide and water.
Law of Constant Proportions
Law of constant proportions states that chemical compound always consists of the sameelements combined together in the same proportion by mass. For example, water is a chemicalrompound that is always composed of hydrogen and oxygen combined in the same constantproportion of 1:8 by mass.
Dalton’s Atomic Theory
Dalton’s Atomic theory of matter states that all matter is made up of very small indivisible particles. Dalton gave his atomic theory of matter in 1805. The various postulates of Dalton’s atomic theory of matter are:
(i) Atoms can neither be created nor destroyed.
(ii) Atoms are of various types.
(iii) Atoms of different elements differ in mass, size and chemical properties.
(iv) All the matter is made up of very small particles called atoms.
(v) Atoms cannot be divided.
(vi) All atoms of given element are identical, having same mass, size and chemical properties.
(vii) The number and type of atoms in a given compound is fixed.
(viii) Chemical combination between two or more elements consists in the joining together of atoms of these elements to form molecules of compounds.
(ix) During chemical combination, atoms of different elements combine in small whole numbers to form compounds.
(x) Atoms of the same elements can combine in more than one ratio to form more than one compound.
Limitations of Dalton’s Atomic Theory
Some of the statements of Dalton's atomic theory are not correct. The following are the limitation of Dalton's atomic theory:
(i) One of the main limitations of Dalton's atomic theory of matter is that atoms were thought to be indivisible. Now it is known that atoms can be divided into smaller particles called electrons, protons and neutrons.
(ii) Another limitation is that this theory assumes that all the atoms of an element have exactly the same mass. Now it is known that atoms of the same element can have slightly different masses. These atoms are called isotopes.
(iii) Another limitation is that this theory assumes that atoms of different elements have different masses. Now, it is known that even atoms of different elements can have the same mass. These atoms are called isobars.
Atoms
Atom is the smallest part of an element that can take part in a chemical reaction. Atoms are extremely small in size and cannot be seen with naked eyes. Even very powerful optical microscopes are incapable of producing a visible image of atoms. Scanning Tunneling Microscope (STM) is the most advanced electron microscope and can produce blurred images of atom. The size of an atom is indicated by its radius called atomic radius and is measured in nanometers. Hydrogen atom is the smallest in size among all other atoms.
These two forms are:
(i) As molecules (ii) As ions
When atoms exist in the form of molecules or ions, they become stable by acquiring stable electronic arrangement.
Molecules
A molecule is an electrically neutral group of two or more atoms chemically bonded together. Atoms are joined together to form molecule by covalent bond. Atoms of same element or different elements combine together to form molecules.
Molecules of elements:
Molecules of elements contain two or more similar atoms combined to form molecule or atoms of same element combined to form molecule. For example, a molecule of oxygen element contains 2 oxygen atoms combined together and is written as \[{{O}_{2}}\] Other examples of molecules of element are hydrogen gas \[({{H}_{2}}),\] nitrogen gas \[({{N}_{2}})\], ozone \[({{O}_{3}})\], sulphur \[({{S}_{8}})\]. The number of atoms present in one molecule of an element is called its atomicity.
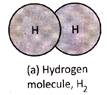
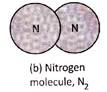
Molecules of elements
Molecules of compounds:
These contain two or more different types of atoms chemically combined together. For example, water (\[{{H}_{2}}O\]) is a compound. It contains two atoms of hydrogen and one atom of oxygen. Other examples of compounds are carbon dioxide (\[C{{O}_{2}}\]), methane (\[C{{H}_{4}}\]), ammonia (\[N{{H}_{3}}\]).
Ions
An ion is a positively or negatively charged atom or group of atoms. Examples of ions are sodium (\[N{{a}^{+}}\]), magnesium ion (\[M{{g}^{2+}}\]), chloride ion (\[C{{l}^{-}}\]). An ion is formed by the loss or gain of electrons by an atom and therefore; ion contains unequal number of electrons and protons. There exists types of ions namely cations and anions.
Positively charged ion is called cation. Cation is formed by loss of one or more electrons by an atom. For example, sodium loses one electron to form sodium ion (\[N{{a}^{+}}\]).
A negatively charged ion is called anion. An anion is formed by the gain of one or more electrons by an atom. For example, chlorine gains one electron to form chloride ion (\[C{{l}^{-}}\]).
The compounds, which are made up of ions, are known as ionic compounds. For example, \[NaCl\]is formed from \[N{{a}^{+}}\] and \[C{{l}^{-}}\]ions, \[CaC{{l}_{2}}\] is formed from \[C{{a}^{2+}}\]and \[C{{l}^{-}}\]ions.
Chemical Formulae of compounds
Chemical formula of a compound indicates the kind and number of atoms of various elements present in one molecule of compound. In the chemical formula of a compound, the elements present are represented by their symbols and the number of atoms of each element are written as subscripts. For example, water consists of two hydrogen atom and one oxygen atom and therefore, the chemical formula of water is\[{{H}_{2}}O\].
Mole Concept
To understand the mole concept, a clear understanding of gram atomic mass and gram molecular mass is required.
Gram atomic mass
When dealing with elements the gram atomic mass or molar mass of element is used. Gram atomic mass is equal to the atomic mass of a substance expressed in grams. The gram atomic mass is simply the atomic mass of the substance expressed with unit gram or g. The gram atomic mass of a substance represents the mass of 1 mole of atoms (\[6.022\times {{10}^{23}}\]atoms) of that substance. Therefore, the number of atoms present in 1 gram atomic mass of any substance is\[6.022\times {{10}^{23}}\]atoms. Atomic mass of hydrogen element is 1, therefore, gram atomic mass will be 1 gram.
Molar mass of a substance is the mass of 1 mole of that substance. The unit of molar mass is grams per mole. Molar mass can be of an element or of a molecular compound. The molar mass of an element is numerically equal to the atomic mass expressed in the units g/mol. Atomic mass of hydrogen element is 1, therefore, molar mass will be 1 g/mol.
Gram molecular mass
When dealing with the molecules of a substance the gram molecular mass or molar mass of substance is used. The gram molecular mass of a substance is the molecular mass of a substance expressed in grams. Molecular mass of oxygen is 32u, therefore, gram molecular mass of a substance is 32 grams. The gram molecular mass of a substance represents the mass of 1 mole of molecules
(\[6.022\times {{10}^{23}}\]molecules) of that substance. Therefore, the number of molecules present in 1 gram molecular mass of any substance is\[6.022\times {{10}^{23}}\]molecules.
Structure of the Atom
Atoms are divisible and have internal structure. Atoms are made of three subatomic particles namely electrons, protons and neutrons.
Thomson’s model of the atom
J.J. Thomson proposed his model of the atom in 1903. According to this model, an atom consists of sphere of positive charge with negatively charged electrons embedded in it. The positive and negative charges in an atom are equal in magnitude that makes the atom electrically neutral and have no overall positive and negative charge.
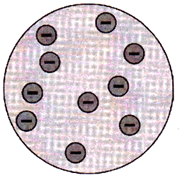
Thomson`s model of the atom
Rutherford’s model of the atom
According to Rutherford's model of atom:
(i) An atom contains a positively charged, dense and very small nucleus containing protons and neutrons. Almost entire mass of an atom is concentrated in the nucleus.
(ii) The nucleus is surrounded by negatively charged electrons. The electrons are revolving around the nucleus in circular paths at very high speed. The circular paths of the electrons are called orbits.
(iii) Atom remains together due to the coulombic force of attraction between the positively charged nucleus and negatively charged electrons.
(iv) The atom is electrically neutral because the number of protons and electrons in an atom is equal.
Bohr’s model of the atom
Neils Bohr model, explains the reason for the stability of atom. According to this model:
(i) An atom is made up of electrons, protons and neutrons. Due to the presence of equal number of negative electrons and positive protons, the atom on the whole is electrically neutral.
(ii) Protons and neutrons are located in a small nucleus at the centre of the atom. Due to the presence of protons, nucleus is positively charged.
(iii) The electrons revolve rapidly round the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represented in numbers 1, 2, 3, 4, 5 and 6 or by letters K, L, M, N, O and P. The energy levels are counted from the centre outwards.
(iv) The maximum number of electrons which can be accommodated in an energy shell (orbit) is given by \[2{{n}^{2}}\]where n stands for the number of the orbit.
(v) There is a limit to the number of electrons, which each energy level can hold. For example, the first energy level or K shell can hold a maximum of 2 electrons, second energy level or L shell can hold maximum of 8 electrons, the third energy level or M shell can hold a maximum of 18 electrons, the bourth energy level or N shell can hold a maximum of 32 electrons.
(vi) Each energy level is associated with a fixed amount of energy; the shell nearest to the nucleus has minimum energy and the shell farthest from the nucleus have the maximum energy.
(vii) There is no change in the energy of electrons as long as they keep revolving in the same energy level, and the atom remains stable. The change in energy of an electron takes place only when it jumps from a lower energy level to a higher energy level or when it comes down from a higher energy level to a lower energy level. When an electron gains energy, it jumps from a lower energy level to a higher energy level, and when an electron comes down from a higher energy level to a lower energy level, it loses energy.
Atomic Number
The atomic number of an element is the number of protons in one atom of an element. For example, one atom of sodium element has 11 protons in it, therefore, the atomic number of sodium is 11. The atomic number of an element is denoted by the letter Z.
Moss Number
The total number of protons and neutrons present in one atom of an element is known as its mass number.
Mass number = no. of protons + no. of neutrons
For example, carbon atom has 6 protons and 6 neutrons, therefore, the mass number of carbon is 6+6 =12. The mass number of an element is denoted by the letter A. Mass number of carbon is 12, therefore, mass number can be written as A = 12.
Valence electrons
The electrons present in the outermost shell of an atom are called valence electrons.
Valency
The number of valence electrons of an element which actually takes part in chemical reactionsis called the valency of that element.
Isotopes
Atoms of the same element which have same number of protons but different number of neutrons inside their nuclei are called isotopes. For example. Isotopes of hydrogen are Protium (\[{}^{1}{}_{1}H\]), Deuterium (\[{}^{2}{}_{1}H\]), Tritium (\[{}^{3}{}_{1}H\])
Isobars
The atoms of different elements with different number of protons (i.e different atomic numbers) but equal sum of the number of protons and neutrons (i.e same mass number) are called isobars. For example,\[{{{}^{40}}_{20}}Ca\], \[{{{}^{40}}_{19}}K\], \[{{{}^{40}}_{18}}Ar\]
You need to login to perform this action.
You will be redirected in
3 sec
