Is Matter Around Us Pure
Category : 9th Class
Is Matter Around Us Pure
Chapter Overview
Matter around us is of two types:
(i) Pure substances
(ii) Mixture.
In this chapter we will study about mixtures and pure substances. Initially, we will discuss about elements and compounds.
A chemical element is a pure substance and it consists of one type of atom distinguished by its atomic number.
Examples of some elements are: helium, carbon, iron, gold, silver, copper, aluminium, hydrogen, oxygen, nitrogen, sulphur, chlorine, iodine, uranium and plutonium.
Our body is also composed of elements but the composition of elements in human body is very much different from that of the Earth's crust, as it can be seen from Table 2.1 given below:
Table 2.1 Elements in Earth’s Crust and Human Body
|
S. No.
|
Elements
|
Percentage by mass
|
|
|
Earth's crust |
Human body |
||
|
1. |
Aluminium |
6.5 |
very little |
|
2. |
Calcium |
3.6 |
1.5 |
|
3. |
Carbon |
0.03 |
18.5 |
|
4. |
Hydrogen |
0.14 |
9.5 |
|
5. |
Iron |
5.0 |
very little |
|
6. |
Magnesium |
2.1 |
0.1 |
|
7. |
Oxygen |
46.6 |
65.0 |
|
8. |
Silicon |
27.7 |
very little |
|
9. |
Sodium |
2.8 |
0.2 |
|
10. |
Sulphur |
0.03 |
0.3 |
A compound can defended as a pure substance made from two or more elements chemically combined together in a definite proportion by mass.
Some examples of compounds are given below-
|
Glucose |
Glycerol |
|
Chloroform |
Sulphuric acid |
|
Methane |
Ethanol |
|
Hydrochloric acid |
Citric acid |
|
Sodium carbonate |
Carbon monoxide |
|
Phenol |
Acetic acid |
Classification of Elements
Elements have been classified on their characteristic properties as metals, non-metals and metalloids.
(i) Metals are good conductor of heat and electricity.
(ii) Freshly cut surfaces of metals have bright shine called metallic lustre.
(iii) Metals are malleable that is they can be hammered to form thin sheets. Some examples of malleable metals are; gold, silver, iron, copper and aluminium.
(iv) Metals are ductile that is they can be drawn into thin and fine wires.
Their properties are usually opposite of those of metals:
(i) Non-metals are poor conductors of heat and electricity.
(ii) Non-metals are not ductile.
(iii) Non-metals are not lustrous.
(iv) Non-metals show a variety of colours.
For example, sulphur is yellow, bromine is reddish brown, chlorine is yellowish green, iodine is violet, carbon is black and phosphorous is red.
They are neither conductors of electricity like metals nor insulators like non-metals, they are semi-conductor. The elements which show some properties of metals and some other prophesies of non-metals are called metalloids. Their properties are intermediate between the properties of metals and non-metals. Metalloids are also sometimes called semi-metals. The important examples of metalloids are: Boron (B), Silicon (Si) and Germanium (Ge).
Differences between Elemnts and Compunds
|
|
Elements |
|
Compounds |
|
1. |
An element is a pure substance which is composed of only one type of atoms. |
1. |
A compound is also a pure substance which is composed of two more type of atoms. |
|
2. |
A monoatomic element cannot be separated by any means of physical and chemical means. |
2. |
A compound can be easily separated into new substances by chemical means. |
|
3. |
There is only limited number of elements in the universe. |
3. |
There are large number of compounds. |
|
4. |
The property of an element is the property of its atom. |
4. |
Properties of constituent atoms and compound formed are quite different. |
3. Mixture
Usually, we deal with a large number of substances in our every day life but majority of them are not pure substances (elements or compounds). They are mixtures of two or more, pure substances.
Mixtures are broadly divided in two major groups: (i) Homogeneous mixtures and (ii) Heterogeneous mixtures.
Homogeneous Mixture
A homogeneous mixture is a mixture where the substances are completely mixed together and have uniform composition throughout.
Different types of homogeneous mixture that may result by mixing different substances have been summarized in Table 2.2.
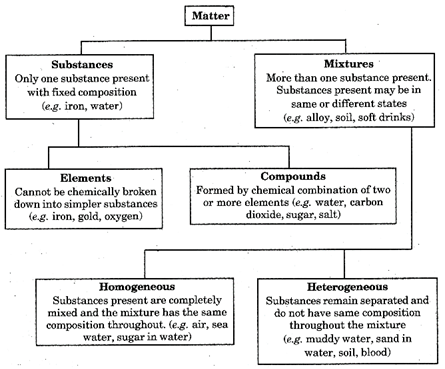
Fig. 3.1 Classification of matter
Table 3.2 Different Types of Homogeneous Mixtures
|
Type of Mixtures |
Description |
Examples |
|
Solid + liquid
Liquid + liquid Gas + liquid
Gas + gas Solid + solid |
Solid dissolves in liquid to form transparent solution. Forms a single transparent mixture. Gas completely dissolves in a liquid to form a transparent solution. Mixture of two or more gases. Some metallic alloys. |
Sugar in water or salt in water, iodine in ethyl alcohol (tincture iodine). Mixture of water and ethyl alcohol. Soda water and any other common soft drink. Air. Brass, bronze. |
Heterogeneous Mixture
A heterogeneous mixture is a mixture where the substances (parts or phases) remain separate and composition is not uniform,
Different types of heterogeneous mixtures that may result by mixing different substances have been summarized in Table 2.3:
Table 3.3. Different Types of Heterogeneous Mixtures
|
Type of Mixture |
Description |
Examples |
|
Suspension |
Solid + liquid. |
Flour in water, river water carrying mud. |
|
Gel |
Liquid trapped in solid. |
Fruit jelly, agar gel. |
|
Emulsion |
Mixture of tiny droplets of one liquid suspended in another. |
Milk. |
|
Aerosol |
Small droplets of liquid or particles of solid dispersed in a gas. |
Clouds (liquid in gas), Smoke (solid in gas) |
|
Foam |
Gas in liquid: small bubbles of gas trapped in liquid |
Shaving foam. |
|
|
Gas in solid: small bubbles of gas trapped in solid |
Polystyrene foam (Thermocol) |
4. Solutions
A solution (a homogeneous mixture) is formed when one or more substances (the solute) are completely dissolved in another substance (the solvent).
(a) Components of a Solution: The substances present in a homogeneous solution are called components of the solution. A solution basically has two components i.e. a solvent and a solute.
(i) Solvent: The component of a solution which is present in large proportion is called solvent. For example: in the Solution of copper sulphate in water, water is the solvent. Similarly, in paints, turpentine oil is the solvent.
(ii) Solute: The component of the solution which is present in small proportion is called solute. For example, in the solution of common salt in water, the common salt is solute. Similarly, in carbonated drinks (soda water), carbon dioxide gas is the solute.
Types of Solutions
(i) Aqueous solution: A solution in which a substance is dissolved in water is called an aqueous solution. In general, inorganic compounds dissolve in water. Since water cam dissolve a wide variety of substances, water is called the universal solvent.
(ii) Non-aqueous solution: A solution obtained by dissolving a substance in a liquid other than water is called a non-aqueous solution. For example; bromine dissolved in carbon tetrachloride, iodine dissolved in ethanol. The common non-aqueous solvents are: methanol, propanone, benzene, carbon disulphide, chloroform etc.
(iii) Solid-solid solutions: All alloys are solid solutions of metals. Brass is a solid solution of approximately 30% of zinc and 70% of copper. In this solid solution, copper (larger component) is solvent and zinc (smaller component) is solute. Similarly, Bell metal is a solid solution of 80% of copper and 20% of tin, in which copper is the solvent and tin is the solute.
(iv) Solid-liquid solutions: Sugar solution is an example, in which sugar is the solute and water is the solvent. Similarly, common salt solution is an example, in which common salt is the solute and water is the solvent. In case of tincture of iodine, iodine is the solute and ethyl alcohol is the solvent.
(v) Liquid-liquid solutions: In case of an alcoholic drink, ethyl alcohol is solute and water is solvent. Similarly, in case of vinegar, acetic acid is solute and water is solvent.
(vi) Liquid-gas solutions: In case of aerated drinks (soda water), carbon dioxide is the solute and water is the solvent.
(vii) Gas-gas solutions: Air is a homogeneous mixtures of two main gases i.e., 78% of nitrogen and 21% of oxygen. In this mixture, nitrogen is solvent and oxygen is solute. Similarly, the petrol fed into the engines of automobiles is a mixture of petrol vapours and air.
Classification of Solution
(i) Saturated solution: A solution, which at a given temperature dissolves as much solute as it is capable of dissolving is said to be a saturated solution.
(ii) Unassured solution: When the amount of solute contained in a solution is less than the saturation level, the solution is said to be an unsaturated solution.
(iii) Super-saturated solution: A solution which contains more of the solute than required to make a saturated solution is called a super-saturated solution.
True Solutions
A solution in which particles of the solute are broken down to such a fine state that they cannot be seen under a powerful microscope is called a true solution.
(i) A true solution is always clean and transparent in light can easily pass through it without scattering.
(ii) The particles of a solute break down to almost molecular size and their diameter is of the order of 1 nm or less.
(iii) A true solution can completely pass through a filter paper as particle size of solute is smaller than the size or the pores of the filter paper.
(iv) A true solution is homogeneous in nature.
(v) In a true solution the particles of solute do not settle down, provided temperature is constant.
(vi) From a true solution, the solute can easily be recovered by evaporation or crystallisation.
Properties of Solutions
(i) The particles of a solution are so small that they are not visible even through a high power microscope.
(ii) A solution is homogeneous in nature.
(iii) A solution is transparent to light and does not scatter light because of very small particle size.
(iv) The particles of solute in solution pass easily through a filter paper.
(v) The properties of solute are retained in solution.
(vi) The diameter of particles in a solution is less than\[{{10}^{-9}}m\].
(vii) The solute particles in solution do not settle on undisturbed position. Therefore, a solution is a stable one.
Concentration of a Solution
Concentration of a solution is defined as the mass of solute present in a definite volume of a solution (which is usually, taken as 1 litre). Concentration of a solution may also be expressed in terms of percent by mass of solute (in gram). This gives the mass of solute per 100 mass units (grams) of solution as shown below:
The concentration of a solute in a saturated solution at a definite temperature is called solubility of that solute in that particular solvent.
The concentration of a solution is expressed in several ways:
(i) Mass by mass percentage of solute in solution.
(ii) Mass by volume percentage of solute in solution.
(iii) Volume by volume percentage of solute in solution.
5. Saturated Solution
A solution obtained by dissolving the maximum amount of the solute in a given quantity of solvent at a constant temperature and pressure is called saturated solution.
6. Super Saturated Solution
It is a solution which contains more mass of solute than a saturated solution can contain at the same temperature. For example, a solution containing 31 g of sodium chloride in 100 grams of water at 293 K is a supersaturated solution.
7. Unsaturated Solution
A solution that contains lesser quantity of the solute than the saturated solution at the same temperature is called unsaturated solution. For example, a solution prepared by dissolving 30 grams (or less) of sodium chloride in 100 grams of water at 293 K is unsaturated solution. We can add more solute so that is can be dissolved in an unsaturated solution.
8. Solubility
It is defined as the maximum amount of solute in grams which can be dissolved in 100 grams of the solvent (i.e., water) at a given temperature to form a saturated solution. It is then called the solubility of the solute in that solvent at that particular temperature.
For example, A maximum of 36 grams of sodium chloride can be dissolved in 100 gram of water at\[20{}^\circ C\], therefore, the solubility of common salt in water at \[20{}^\circ C\]is 36 grams.
Units of Solubility
Solubility is always expressed as the mass of solute dissolved in 100 mass units of solvent resulting in a saturated solution. It is also expressed as mass of solute per unit volume of solution.
9. Suspension
A heterogeneous mixture of insoluble particles of solute, spread throughout a solvent, is called a suspension.
Examples:
(i) Muddy water in which particles of sand and clay are suspended in water.
(ii) Slaked lime suspensions used for white washing has particles of slaked lime suspended in water.
(iii) Paints in which the particles of dyes are suspended in turpentine oil.
Properties of a Suspension
(i) The size of the particles is more than\[{{10}^{-5}}cm\].
(ii) The particles of suspension can be separated from solvent by the process of filtration.
(iii) The particles of suspension settle down, when the suspension is left undisturbed.
(iv) A suspension is heterogeneous in nature.
(v) Suspension is a heterogeneous system of solid particles in a liquid.
(vi) In a suspension the solid particles dispersed in the medium can be seen by the naked eye.
(vii) Particles of a suspension scatter a beam of light passing through it and make its path visible.
(viii) Solid particles of a suspension do not pass through the pores of filter paper.
10. Colloidal Solutions
A colloidal is a heterogeneous system of two or more substances in which the size of the dispersed particles is greater than the size of the solute particles of a true solution but smaller than the size of the particles of a suspension.
Examples of Colloidal Solutions:
Characteristics of Colloidal Solution
(i) The size of colloidal particles is between \[{{10}^{-7}}cm\]and\[{{10}^{-5}}cm\].
(ii) The particles of colloidal solution are visible under a powerful microscope.
(iii) The particles of a colloidal solution do not settle down with the passage of time.
(iv) The particles of a colloidal solution can easily pass through filter paper.
(v) The particles of a colloidal solution scatter light i.e., when strong beam of light is passed through the colloidal solution, the path of beam becomes visible.
(vi) Colloidal solution are not transparent but translucent in nature.
(vii) The particles of a colloidal solution are electrically charged.
(viii) The colloidal solutions are heterogeneous in nature.
Classification of Colloids
The colloids are classified according to the state of dispersed phase (Solid, liquid or gas) and the state of dispersing medium. A few examples are shown in following table:
|
Dispersing medium |
Dispersed phase |
Type of Colloidal Solution |
Examples |
|
Gas |
Solid |
Aerosol |
Smoke |
|
Liquid |
Solid |
Sols |
Glue, paints |
|
Solid |
Solid |
Solid sols |
Ruby glass, gem stones |
|
Gas |
Liquid |
Aerosol |
Fog, cloud in sky |
|
Liquid |
Liquid |
Emulsions |
Milk, oil drops |
|
Solid |
Liquid |
Gels |
Cheese, butter, shoe polish |
|
Liquid |
Gas |
Foam |
Cream, froth |
|
Solid |
Gas |
Solid foam |
Foam rubber, stone |
Different examples of Colloidal Syatem
Uses of Colloids
Properties of Colloidal System
A colloidal system shows the property of Brownian motion, Tyndall effect and electro phoresis.
11. Brownian Motion
Random motion in zig-zag path followed by colloidal particles is called Brownian motion. This type of motion of colloidal particles is called Brownian motion after the name of its discover Robert Brown,
The basic cause of Brownian motion is due to the collisions between the molecules of the dispersion medium and the colloidal particles.
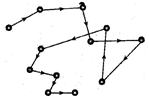
Fig. 11.1: Brownian motion
12. Tyndall Effect
When a strong beam of visible light is passed through a true solution taken in a beaker and placed in a dark container the path of the light is dark. But, if the light is passed through a colloidal solution the path of light becomes visible. This phenomenon is called Tyndall effect.
It is caused due to scattering of light by the colloidal particles.
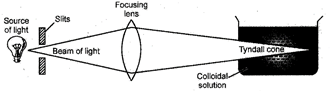
Fig. 12.1 Tyndall effect
Examples of Tyndall Effect
(1) The blue colour of sky, visibility of tails of comets and twinkling of stars are explained on the bases of Tyndall effect.
(2) Tyndall effect is observed when a fine beam of light enters a dark room through a small hole.
(3) Tyndall effect can also be observed in a dense forest in the morning. This happens because the mist contains very small droplets of water dispersed in air which scatter the sunlight.
13. Electro Poesies
When we apply electricity through a colloidal system, the particles move either towards the positive or the negative electrode. This migration of colloidal particles towards the oppositely charged electrode under the influence of an electric field is called electrophoresis,
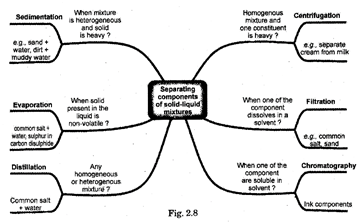
14. Separation of mixtures
The following two factors decide the best possible techniques to be adopted for separation:
(i) The type of mixture
(ii) The component which you want to collect.
How Can We Obtain Colored Component (Dye) From Blue Black Tank? (Evaporation)
The separation of liquid (solvent) and solid (solute e) from a solution is done by removing the liquid (solvent) by heating or by solar evaporation. By evaporation you can recover the solute component only in solid or powder from. If the solvent is inflammable you cannot use flame for heating instead you can use an electrical heating system and an oil or water both.
How Can We separate A Mixture of Two emmiscible Liquids? (Separation using a Separating Funnel)
The mixture of two immiscible liquids (i.e., the liquids that do not mix, such as oil and water) can be separated by using a separating funnel. The mixture is placed in separating funnel and allowed to stand for sometime. When the two layers of liquids are separated, the denser liquid which is in the lower part, is first collected by opening the stop-cock. This method is very useful in industries.
Application of separating Funnel
(i) To separate out a mixture of oil and water or mixture of two immiscible liquids, like benzene and water, carbon tetrachloride and water etc.
(ii) To separate out molten slag and molten iron in the blast furnace, during extraction of iron.
(iii) In extraction of organic compounds present in aqueous solutions using water immiscible organic solvent, like chloroform, benzene, ether etc.
15. How Can We Separate Cream From Milk? (Centrifugation)
Centrifugation is based on the principle when a machine rotates with a very high speed then the denser solid particles settle down faster leaving behind the lighter particles or we may say the lighter particles stay on the surface of the liquid while the heavier particles settle down at the bottom of the liquid. The machine used to generate centrifugal force is called the centrifuging machine,
Applications of Centrifugation
How can We separate of Mixture of salt And Ammonium Chloride? (Sublimation)
Some solid substances, when heated change directly into vapours. The process of converting a solid substance from the solid state to its vapour by heat and allowing it to solidify again is called sublimation. The solid obtained by sublimation is called sublimate. Sublimation is described by the following equation:
\[SolidVapour\]
For example, the components of a mixture of iodine and sand can be separated by sublimation. Some examples of solids which sublime are ammonium chloride, camphor, naphthalene and anthracene.
Activity to show sublimation: We take a mixture of solidified ammonium chloride and salt in a china dish and cover it by inverting a glass funnel. We place the china dish on a wire gauze and tripod stand and heat by a Bunsen burner. Ammonium chloride undergoes sublimation producing violet vapours.
Chromatography
The term chromotography has been taken from the word chroma (Kroma a Greek word), which means colour. The process of separating coloured components of a mixture is known as chromatography.
Basically, the process of separation of the components of a mixture based on the difference in adsorption of different components on the surface of a solid is known as chromatography and use of the chromatographic technique by using a strip of filter paper is called paper chromatography.
16. Types of Chromatography
Paper Chromatography
In paper chromatography, a filter paper strip acts as stationary phase and a suitable solvent {i.e., water) or an organic solvent is used as mobile phase. A fine spot of the sample is placed on the chromatographic paper.
The solvent diffuse through the filter paper and moves upward. When solvent moves it carries with it the substances of the mixtures present in the spot on the filter paper. During this process the substances which are absorbed strongly on the filter paper move with slow speed. On the other hand, the substances which dissolve strongly in the solvent move with high speed.
After this we can see different spots on the filter paper as the solvent moves upward.
Finally, after 10 minute the chromatographic paper is removed from the gas jar and left to dried. At the final step the various spots and solvent on the paper are marked.
Application of Chromatography
(i) Chromatographic technique is used to separate colours in a dye.
(ii) Chromatographic technique is used to separate pigments from natural colours.
(iii) This technique is also used to separate drugs from blood.
17. Distillation
The method of distillation is used to separate a liquid from a solution of a homogeneous mixture. The distillation is a process in which a liquid or mixture of liquids is boiled in a distillation flask. The vapour is condensed by passing through a water-cooled tube called condenser and collected as liquid called distillate. In case of a solution of two miscible liquids (the liquids which can be mixed completely) the separation is based on the fact that the liquids will have different boiling points and there is a wide difference between the boiling points of the two liquids.
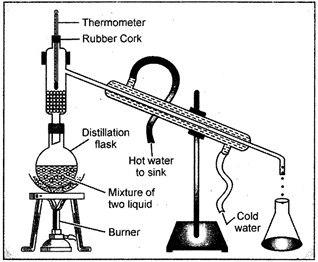
Fig. 17.1: The Distillation apparatus
Applications of simple Distillation
18. Fractional Distillation
This method is used for separating the components of a mixture containing two or more miscible liquids whose boiling points differ by at least\[15{}^\circ C\]. The liquid mixture is taken in a round-bottomed distilling flask which is connected to a fractionating column. The fractionating column is in the form of tube. Fractionating column is used to increase the cooling surface area and to provide obstructions to the ascending vapours and descending liquid. The side tube of the fractionating column is fitted with a Liebig's condenser. Through the outer tube of the Liebig's condenser water is circulated continuously in order to condense the vapours. To prevent bumping a few small pieces of porcelains are added to the distillation flask.
Bunsen burner is used to heat the distillation flask.
On heating the temperature of the mixture rises gradually and the liquid starts boiling. The vapours of the low boiling component leave the fractionating column from the exit near the top while the vapours of the high boiling component get condensed in the fractionating column and return to the distillation component flask. The vapours of the high boiling component enter the Liebig's condenser and get condensed. The distillate consisting of pure but more volatile component is collected in the receiver. The high boiling component distills afterwards at a higher temperature.
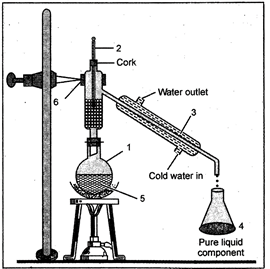
Fig. 18.1.: Fractional distillation
Application of Fractional Distillation
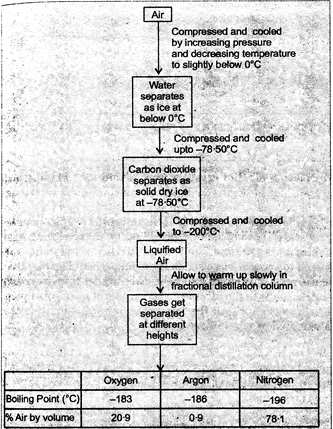
Fig. 18.2. Flow chart for separation of the components of air
Benzene (boiling point \[80{}^\circ C\]) and toluene (boiling point \[110{}^\circ C\]).
How Can We Obtain Different Gases From Air
Clean air is a homogeneous mixture of various gases, such as nitrogen, oxygen, carbon dioxide, water vapour, gases and other noble gases. As these gases have different freezing and boiling points, these gases can be separated by liquefaction of air followed by fractional distillation of liquid air in a specially designed column. The flow chart of this process is shown in figure 2.6
Experimental arrangement for separation of the components of air
Firstly, the air is passed through filters so that dust particles are removed. In filter the dust free air is compressed and cooled. Below the temperature\[0{}^\circ C\], water separates as ice. On further cooling (up to \[-78.5{}^\circ C\]), carbon dioxide separates as snow. Now air containing nitrogen, oxygen and argon is cooled upto -\[200{}^\circ C\]. At -\[200{}^\circ C\] the gases change into liquid. The liquid air is distilled (warmed from \[-200{}^\circ C\] to\[-196{}^\circ C\]) so that nitrogen changes into gas leaving behind the liquid oxygen and argon. The nitrogen gas is collected in fractional distillation from \[-196{}^\circ C\]to -
\[183{}^\circ C\]so that oxygen is changed into gaseous state. Oxygen gas is collected in separate cylinders under pressure. Argon which is free from other components is collected out from fractional distillation column.
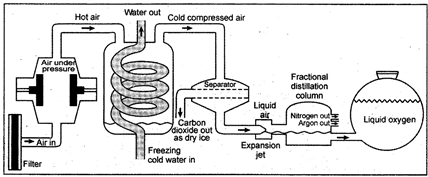
Fig. 2.7: Separation of components of air
19. Crystallization
Crystallization is a process of formation of solid crystals from a solution. The method of crystallization for separating solid from liquid begins by evaporating the liquid. However, in crystallization, the evaporation is stopped when the solution is concentrated enough. The concentrated solution thus produced, is allowed to cool slowly to form crystals which can be separated by filtration. Mishri (sugar crystals) is produced by crystallization from concentrated sugar solution. An impure solid is purified by crystallization. If the solubility of two solids are different, fractional crystallization is used to separate two solids. The impure solid is dissolved in a suitable solvent to get its nearly saturated solution at a temperature higher than the room temperature. The cooling of such a solution leads to the formation of crystals of pure substance.
The solution left behind is called the 'mother liquor'. All the impurities are left behind in the mother liquor. Impure common salt is purified by crystallization.
20.Water Purification in Water Works
In cities drinking water is supplied through pipes. For this purpose water from the river is pumped in big reservoirs. A simplified sketch of the water supply system is shown in figure 2.15.
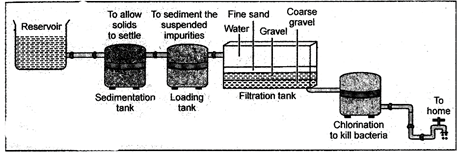
Fig. 20.1: Water purification system in water works
The various steps of water purification are as follows:
A calculated concentrated solution of chlorine is mixed with aerated water. Chlorine acts as a disinfectant and kills the harmful disease causing micro-organisms. This process of killing the bacteria and micro-organisms from water is called sterilization of water.
After all these processes pure water thus obtained is stored in large tanks and supplied to cities through pipes. We thus get pure water in our taps.
21. Separation based on magnetic properties
In a mixture of magnetic and non-magnetic substances, the magnetic substance can be separated by using a magnet. For example, you will be able to separate iron granules, which are magnetic, from non-magnetic substances, like sand, sugar, saw dust etc. see figure 2.16. In industries this method is used to separate iron materials from non-magnetic materials by using large electromagnets, e.g. separation of iron ore.
22. Physical And Chemical Change
A physical change is a temporary change in which no new substance is formed and substance undergoing the change does not lose its identity or we may say the composition of the original sub- stance is not altered after the change, although, certain specific physical properties may be changed.
Examples of Physical Changes
Characteristics of A Physical Change
Chemical Change
It is a permanent change in which the identity of the original substance is changed and new substance or new substances are formed. This new substances has different property from the original one.
Since new substances with different molecules are formed during chemical reactions, it is not easy to get back the original substances. Most chemical changes are, therefore, permanent and irreversible.
23. Examples of Chemical Change
Characteristics of Chemical Change
Chapter at a Glance
You need to login to perform this action.
You will be redirected in
3 sec
