Matter In Our Surroundings
Category : 9th Class
Matter in Our Surroundings
Matter
Matter is anything that has mass and occupies space. Examples of matter are iron, wood, oil, kerosene, petrol, rock, minerals, water, air, coal, etc. Matters are classified on the basis of physical and chemical properties. On the basis of physical properties, matter is classified as solid, liquid and gas. On the basis of chemical properties, matter is classified as elements, compounds and mixtures. Matter is made up of particles.
Characteristics of Particles of Matter
The various characteristics of particles of matter are:
Classification of Matter on the Basis of Their Physical Properties
On the basis of physical properties, all the matters are classified into three groups namely solid, liquid and gas. In other words, it can be said that matter exists in three physical states namely solid, liquid and gas.
States of Matter
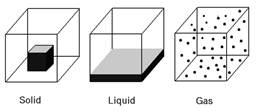
States of Matter
The following are the properties of three states of matter:
Solid
The various properties of solids are:

Stone (Solid)
Liquid
The various properties of liquids are:

Water (Liquid)
Gas
The various properties of gases are:

Air (gas) balloon
Changing the State of Matter
The three physical states of matter can be changed by changing the temperature and pressure.
Changing the State of Matter by Changing Temperature
The temperature can be increased by heating to change solid into liquid and liquid into gas. The temperature can be decreased by cooling to change gas into liquid and liquid into solid.
The temperature at which a solid substance melts at atmospheric pressure is called melting point of the substance. The higher the force of attraction between particles of a solid substance, the greater is the melting point of that substance. On heating solid substance, the particles of substance vibrate vigorously. When the melting point is reached, the particles of a solid have sufficient amount of kinetic energy to overcome the force of attraction holding the particles at fixed position. Therefore, the solid melts and turns into liquid state.
The temperature, at which a liquid changes its state to vapours at atmospheric pressure, is called boiling point of the liquid. On heating liquid substance, the particles of substance vibrate vigorously. When the boiling point is reached, the particles of a liquid have sufficient amount of kinetic energy to overcome the force of attraction holding the particles at its position. Therefore, the liquid boils and turns into gaseous state.
On cooling, particles of gaseous substance lose kinetic energy, therefore; they vibrate slowly and move closer until the particles become attracted towards each other and form a liquid.
On cooling particles of liquid substance lose kinetic energy, therefore; they stop moving and move closer until the particles become attracted towards each other and acquires a fixed position to turn into a solid.
The heat given to a substance to change its physical state is called latent heat. Normally heat given to a substance rises the temperature of the substance, but in case when change in physical state of substance is involved, there is no rise in the temperature of the substance. The amount of heat energy required to change the state of a substance is called its latent heat. The latent heat does not increase the temperature of a substance because this heat is consumed for overcoming the forces of attraction between the particles of a substance during the change of state. Therefore, the kinetic energy of the particles of the substance is not increased. The latent heat can be of two types. They are:
Latent Heat of Fusion
The latent heat of fusion or melting of a solid is the amount of heat in joules required to convert 1 kilogram of solid to liquid without causing any change in temperature. For example, the latent heat of fusion of ice is 334 KJ per kg. Which is the amount of heat that converts ice into water without causing any increase in temperature.
Latent Heat of Vaporization
The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of liquid to vapour or gas without causing any change in temperature. For example, the latent heat of vapourisation of water is 2258 KJ per kg which is the amount of heat that converts water into vapours without causing any increase in temperature.
Sublimation
When a solid directly changes into gas or vapour on heating and vapours changes directly to solid substance, on cooling, is called sublimation. For example, ammonium chloride undergoes sublimation. On heating, ammonium chloride changes directly into ammonium chloride vapour. When solids that undergo sublimation are heated, their particles move very quickly that they separate completely to form vapour or gas. When the vapour or gas is cooled, the particles of gas slow down very quickly and acquire fixed position to from a solid. Solid carbon dioxide or dry ice also undergoes sublimation. When dry ice is heated, it sublimes to form carbon dioxide gas. Another common example of substance that undergoes sublimation is naphthalene balls. Naphthalene balls disappear with time without leaving any residue behind. Few substances that undergo sublimation are ammonium chloride, iodine, camphor, naphthalene and anthracene.
Changing State of Matter by Changing Pressure
The physical state of matter can also be changed by changing the pressure. Gases can be changed into liquids by increasing pressure along with decreasing temperature. Similarly some solids such as solid carbon dioxide can be changed into gases on decreasing the pressure and increasing the temperature.
Classification of Matter on the Basis of Chemical Properties
On the basis of chemical properties the matter is classified into two Types namely pure substances and mixtures. The pure substances can be of two type namely elements and compounds.
Pure Substance
Pure substances are made of only one kind of particles. The particles can be atoms or molecules. For example, water is made up of only water molecule and therefore, water is a pure substance. Another example is sodium element which is made up of only sodium atom and therefore, is a pure substance. All elements and compounds are pure substances because they contain only one kind of particles. Thus, all the elements such as hydrogen, nitrogen, bromine, iodine, silver, etc. are pure substances. Similarly, all compounds such as water, carbon dioxide, hydrochloric acid, camphor, etc. are pure substances.
An element is made up of only one kind of atom. An element is a substance that cannot be broken down into two or more-simpler substances by the usual chemical methods of applying heat, light or electric energy. The elements can be in solid, liquid or gaseous state at room temperature.
On the basis of the properties of elements, they can be classified as:
Metals
Metals are elements that are malleable, ductile and conduct electricity. Metals have tendency to lose electrons. Some of the metals are zinc, silver, iron, potassium, mercury, uranium, etc. All metals are solids except mercury, which is a liquid.
The properties of metals are:
Non Metals
Non-metals are elements that are neither malleable nor ductile and do not conduct electricity. Non-metals have tendency to gain electrons. All non-metals exist in solid or gaseous state except bromine which exists in liquid state at room temperature. Some examples of non-metals are neon, argon, krypton, xenon, etc.
The properties of mm metals are:
Metalloids
Few elements have some properties of metals and non-metals. For example, few elements have appearance similar to metals but are brittle like non-metals. These elements are neither conductors of electricity like metals nor insulators like non-metals, instead they are semiconductors. These elements that show properties of both metals and non-metals are called metalloids. They have properties that are intermediate between the properties of metals and non-metals. They are sometimes called semi metals. Examples of metalloids are boron (B), silicon (Si), germanium (Ge).
Compounds
Compounds are the substances made up of two or more elements chemically combined in a fixed proportion by mass. For example, water \[({{H}_{2}}O)\] is a compound made up of two elements hydrogen and oxygen, chemically combined in fixed proportion of \[1:8\] by mass (Atomic masses:\[H=1u\], \[O=16u\], therefore \[{{H}_{2}}:O=2u:16u\]or\[1:8\]). Other compounds are salt or sodium chloride\[(NaCl)\], ammonium chloride\[(N{{H}_{4}}Cl)\].
Mixtures
A mixture consists of two or more elements or compounds that are not chemically combined together. For example, sugar solution, air and milk are examples of mixture. In sugar solution, the sugar and water are physically mixed without any chemical reaction between sugar and water. Similarly air constitutes mixture of different gases and milk consists of fat, water and proteins.
Mixtures can be of two types:
Solution
Solution is a homogeneous mixture of two or more substances. Some common examples of solutions are salt solution, vinegar, metal alloys, air, etc.
Properties of solution are:
(i) A solution is a homogeneous mixture.
(ii) A solution has solute particle, which is extremely small in size. It is less than \[{{10}^{-9}}m\]in diameter.
(iii) The particles of a solution cannot be seen even with a microscope.
(iv) The particles of a solution pass through the filter paper. Therefore, different particles of solution cannot be separated by filtration.
(v) The particles of solution do not get separated, even after the solution is kept for some time.
(vi) A true solution does not scatter light, due to the very small size of its particles.
Different types of solutions are:
Suspension
Suspension is a heterogeneous mixture in which the small particles of solute are spread throughout a liquid without dissolving in it. For example, chalk and water mixture, Paints, sand and water mixture.
Properties of suspension are:
(i) A suspension is a heterogeneous mixture.
(ii) Size of solute particles of suspension is in the range of \[{{10}^{-7}}\]to \[{{10}^{-5}}m\]diameter.
(iii) The particles of a suspension can be seen easily.
(iv) The particles of suspension can be separated by filtration.
(v) The particles of suspension separate, after the solution is kept for some time. Therefore, suspensions are unstable.
(vi) Suspension scatters beam of light passing through it due to quite larger size of its particles.
Colloids
Colloids consist of particles whose size is intermediate between those in true solutions and those in suspensions. When seen with naked eye colloids appear homogeneous, but are found heterogeneous when observed through a high power microscope. Some of the examples of colloids are soap solution, starch solution, blood, milk, etc.
The properties of colloids are:
(i) A colloid appears to be homogeneous, but it is heterogeneous.
(ii) The size of particles in a colloid is bigger than those in a true solution but smaller than those in a suspension. It is between \[{{10}^{-9}}\] to \[{{10}^{-7}}m\]diameter.
(iii) The particles of most of the colloids are visible under powerful microscope.
(iv) The particles of colloids cannot be separated by normal filtration.
(v) The particles of colloids do not separate out if the colloid is kept for some time.
(vi) The colloid scatters a beam of light passing through it.
You need to login to perform this action.
You will be redirected in
3 sec
