Natural Resources
Category : 9th Class
Natural Resources
Chapter Overview
Our earth is the only planet which has all the essential conditions required for the existence and survival of life. Life on earth is dependent on many factors such as an optimum temperature, water, food and air. The resources available on earth and the energy from the sun are necessary to meet the basic requirements of all life forms on the earth.
What are these resources on the Earth?
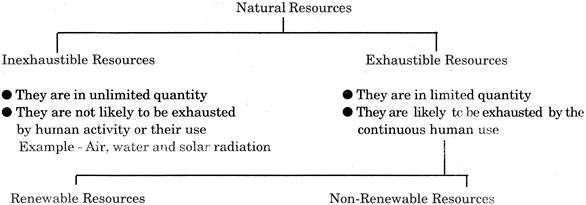
All the varieties of substances that we get from Earth and nature to meet our basic needs are called Natural Resources. The word resource means a source of supplying a material generally held in reserve. These materials present in nature are air, water, soil, minerals petroleum, coal, animals and plants. These all are obtained from nature, thus, they are known as natural resources. Some of the related terms are as follows:
(a) Lithosphere: It is outer solid crust of earth, also called land. Its upper thin weathered layer is known as soil.
(b) Hydrosphere: It is water component of earth. 75% of the surface of the earth is covered by water, in form of oceans, rivers, lakes, ponds, dams etc. Another component of hydrosphere is underground water.
(c) Atmosphere: The air that covers the whole of earth like a blanket is known as atmosphere.
(d) Biosphere: Life forms are found where all of the three resources (i.e. lithosphere, hydrosphere and atmosphere) are found and interact with each-other. This life supporting zone of the earth ig known as Biosphere. Biosphere has two types of components biotic and abiotic.
(i) Biotic components of biosphere are the living things. Various micro-organisms, plants, and animals form the biotic components of biosphere.
(ii) Abiotic components of biosphere are the non-living things. Air, water, soil, temperature, minerals, metals etc. form the abiotic components of the biosphere.
Both the types of components provide resources to meet the basic requirements of life forms. However the energy required for sustaining life is ultimately obtained from the sun.
|
They can replenish themselves by quick recycling and replacement within a reasonable time. |
They cannot replenish themselves by recycling and replacement |
|
|
They will ultimately get exhausted. |
|
They can last forever if their use is limited. |
Only non-living resources. |
|
Both living and non-living resources. |
Increased consumption will result in quicker exhaustion. |
|
Availability can be enhanced by increasing replenishment. |
Example: Fossil fuels, coal, petroleum minerals etc. |
|
Example: Soil, forests, wild life. |
|
Air is a mixture of many gases like nitrogen, oxygen, carbon dioxide, and water vapours.
Air forms a protective transparent envelope or atmosphere around the earth. Air is dense near the surface of earth. It becomes progressively rarefied with height. The height to which air extends is 500 km. It does not escape into space, being held to earth by gravity.
Composition of Air
|
Nitrogen \[({{N}_{2}})\] |
78.08% |
|
Oxygen \[({{O}_{2}})\] |
20.94% |
|
Carbon dioxide \[(C{{O}_{2}})\] |
0.03% |
|
Argon |
0.93% |
There are Traces of Neon (Ne), Helium (He), Krypton (Kr), Xenon (Xe), Ozone\[({{O}_{3}})\]methane \[(C{{H}_{4}})\]Nitrogen oxides \[(N{{O}_{x}})\]Sulphur dioxide \[(S{{O}_{2}})\], Carbon monoxide (CO), Ammonia (NHs) in air.
Atmosphere of earth is divisible in four main concentric layers that differ in density, temperature, composition and properties. These layers are Troposphere, Stratosphere Mesosphere and Thermosphere.
Troposphere is the lowest layer of atmosphere which extends upto a height of 8-16 kms.
Many climatic events such as air movements, clouds formation, lightening, thundering etc.
occur in troposphere. Lower part of troposphere is the reservoir of gases that are essential for the life e.g., oxygen and carbon dioxide. It also contains dust particles, spores and pollens.
Stratosphere extends from upper part of troposphere upto a height of 16 to 50 kms. It has a protective ozone shield that protects us from harmful UV radiations of sunlight. Layer of ozone is called ozonosphere which lies at a height of 11-16 km above poles and 23-25 km above equator.
Mesosphere extends between 50 and 85 kms and next to stratosphere. Between 85-500 kms, the atmosphere is called thermosphere because there is progressive increase in temperature.
lonisation of gases takes places in this layer due to high temperature and high energy radiations.
The layer of ionised particles is known as ionosphere. It is radio-reflective and is important for telecommunication on earth.
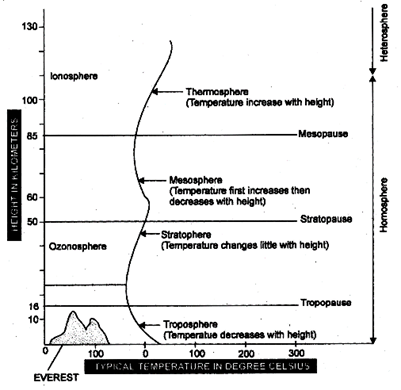
Fig. 2.1: Main layers of the atmosphere. These are shown between solid lines. The vertical wavy line depicts temperature variation with increasing height.
Air is called the breath of life, as life cannot exist without air. Even the composition of the air is largely due to activity of living beings on earth. No life has been found on the planets Venus and Mars where the atmosphere is made up of upto 95-97% carbon dioxide. Hence, we can say that \[C{{O}_{2}}\]is the major component of these planets.
Functions of Atmosphere or Air
(a) Oxygen: Atmosphere contains oxygen, which is utilized in the following ways—
(i) Aerobic respiration: Oxygen is required by all the eukaryotic cells and many prokaryotic cells for breaking down glucose molecules and get energy for their living activities. This results in the production of carbon dioxide. Such organisms which require oxygen for obtaining energy called aerobic organism and this process is aerobic respiration.

(ii) Combustion: It is another process that requires consumption of oxygen to release carbon dioxide. It includes burning of wood, fossil fuel (coal, petroleum, and natural gas), organic wastes etc. by man in various ways. Forest fires also consume oxygen to release carbon dioxide and lot of energy.
(b) Carbon dioxide: Although \[C{{O}_{2}}\]is released tremendously by the above processes, but it is also consumed or fixed continuously in the following two ways—
Green plants convert \[C{{O}_{2}}\]into glucose in the presence of sunlight. This process is called Photosynthesis.
\[\underset{(Carbon\,dioxide)}{\mathop{6C{{O}_{2}}}}\,+\underset{(Water)}{\mathop{12{{H}_{2}}O}}\,\] \[\underset{(Glucose)}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{(Water)}{\mathop{6{{H}_{2}}O}}\,+\underset{(Oxygen)}{\mathop{6{{O}_{2}}}}\,\]
(ii) Many marine animals use carbonates dissolved in sea water to make their shells.
We know that the air is a bad conductor of heat and serves as a blanket on earth. The following factors of the atmosphere help in controlling the climate of particular region so that the living organisms can survive in it.
(i) Air is bad conductor of heat: The atmosphere keeps the average temperature of the earth fairly constant during the day and even during the course of the whole year. The atmosphere does so by preventing the sudden increase in temperature during the day light hours. Further, during the night, it slows down the escape of heat into the outer space thereby preventing excessive cooling during the night. All this happens because the air is a bad conductor of heat.
(ii) Distance from the Sun: Distance of the moon from the sun is the almost same as that of the earth. But, the temperature on the surface of the moon ranges from \[-190{}^\circ C\]to\[110{}^\circ C\]. In other words, it cools down to - 190°C during night and rises to a very high of 110°C during day. This happens because there is no atmosphere on the moon. Hence, the life is not possible on the moon.
(iii) Atmosphere as a Blanket: The atmosphere acts as a blanket for the earth. The ozone layer of the atmosphere absorbs most of the harmful ultraviolet radiations coming from the sun. The excessive heat and sun rays are reflected back into the outer space by dust particles, clouds, water vapours etc.
The Movement of Air: Winds
Many times, after a hot day, we would have felt cool breeze in the evening. Sometime, it rains after few days of really hot weather. Following questions may strike our mind:
(i) What causes the movement of air?
(ii) What decides whether this movement will be in the form of a gentle breeze, a strong wind or a terrible storm?
(iii) What brings the rain?
All these phenomena are the result of change that takes place in the atmosphere due to heating of air and the formation of water vapour.
Water vapours are formed due to the heating of water bodies by Sun's rays as well as due to the activities of living organisms (i.e. transpiration from plants, activities of animals and evaporation from moist surface). Water vapours rise upward and condense. Condensation of water vapours liberates a lot of energy which may cause cyclones and thunderstorms.
Heating of air which occurs because the atmosphere can be heated from below by the radiation that is reflected back or re-radiated by the land or water bodies. In fact the sun-rays fall on the earth, some of them are absorbed and majority of these are reflected back or re-radiated by the land and water bodies. These reflected sun-rays or solar radiations heat up the atmosphere from below as a result, convection currents are setup in the air. But since the land gets heated faster than the water, the air over land also get heated faster than the air over water bodies. Hot air over land rises upwards thereby producing an area of the low air pressure. Air from area of high pressure will move towards this area of low pressure producing breeze or wind.
Let us perform an activity to understand the nature of convection currents.
Air Movement in Coastal Areas
In coastal areas, there is a regular flow of cool breeze from sea towards the land during the day time. During night, there is a reverse flow of air from land to sea. It is because, during day time land gets heated faster than water. Re-radiation from land heats the air above it. The hot air rises and creates low pressure area. Sea water is not heated so rapidly. Air above sea remains comparatively cool. It has a higher pressure as compared to air over land. Therefore, cool breeze (cooler air) present over sea moves towards land where low pressure exists.
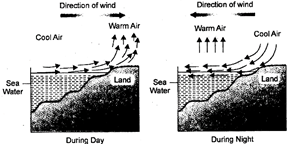
Fig. 2.2: Movement of wind from sea to land during day and from land to sea during night in coastal areas
During night, reverse flow of air occurs. Land cools down rapidly. Also air over it becomes cool. Sea water cools slowly. Air above it is hotter with lower air pressure as compared to air pressure over land. Therefore, cool breeze present over land moves towards sea.
Factors Influencing Movements of Air
Depending upon the difference in energy levels and air pressure, there occurs various type it atmospheric phenomena such as breeze, wind storm, thunderstorm, monsoon rains, cyclones the factors controlling these phenomena are:
(i) Uneven heating of land in different parts of earth.
(ii) Difference in heating and cooling of land and water.
(iii) Rotation of earth.
(iv) Vapourisation and condensation of water vapours.
(v) Pressure of mountain ranges in the paths of wind.
Cloud formation and Rain
There are various steps to understand the formation of clouds resulting into rain.
(i) The water enters into the atmosphere through two routes:
(a) During the day time, a large amount of water evaporates and goes into the atmosphere.
This happens due to heating of the water bodies.
(b) Some amount of water also comes into the atmosphere because of many biological Processes (i.e. Transpiration etc.)
(ii) The air gets heated during day time. The hot air along with water vapours rises up in the atmosphere.
(iii) At a height the air expands and becomes cool. This cooling causes the water vapours in the air to condense in the form of tiny droplets. This condensation of water is helped if some particles (usually dust and other suspended particles) could act as the nucleus for these droplets to form around. This produces clouds and they float along air currents.
(iv) Further condensation of these water droplets leads to increase in their size. When the drops become big and heavy, they start falling down in the form of rain. But if the temperature of the air at an area, is very low, precipitation may occur in the form of snow (Precipitates falling from clouds in the form of ice crystals), Sleet (a mixture of rain and snow) or hail (Precipitates of ice pellets when there are strong rising air currents).
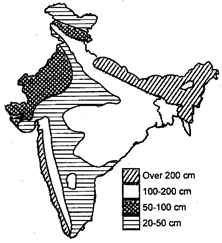
Fig. 2.3: Rainfall zones of India
The circulation of water in the form of water vapours, clouds, rain and snow forms the water cycle.
Rainfall pattern of an area is therefore, decided by availability of water vapours, regions of low pressure, prevailing wind, condensation of water vapours and direction of mountains. In our country most of the rainfall is brought out by South-West monsoon and to a smaller extent by Intensity and periodicity of rainfall differ from region to region. As far as the amount of rainfall is concerned, India has four zones—Arid zone (20-50 cm). Semi-arid zone (50-100 cm) Intermediate zone (100-200 cm) and Wet zone (more than 200 cm (eg. North East India, Western Ghats).
To understand the formation of clouds, rains and the factors influencing these climatic changes, following activities are necessary to be performed.
In a healthy environment all components should be balanced. But increase in human population and great advancement in industry and technology in the recent past have damaged the balanced and healthy environment. The imbalance in our environment has given rise to various environmental problems and majority of them are related to air, water and soil pollution- Clean and pure air is very essential for the health and survival of man and other living beings. However, with the progress in man's living standards, air has become more polluted.
Air pollution may be defined as an undesirable change in the physical, chemical or biological characteristics of the air that harms human life and other living beings.
In other words addition of unwanted and harmful particles in the air or increase in the quantities of constituents of air beyond the normal level that affects the living organism is called air pollution. The agents or substances that cause air pollution are called air pollutants.
Common air pollutants are gaseous pollutants and particulate matter. Gaseous pollutants include oxides of carbon (e.g. carbon monoxide, excess of carbon dioxide), sulphur dioxide, oxides of nitrogen (e.g. \[NO,N{{O}_{2}},{{N}_{2}}O\]etc.) hydrocarbons, chlorofluorocarbons (CFCs), hydrogen sulphide, methane, ammonia etc. Particulate matter is also called suspended particulate matter (SPM) because it remains suspended in air for a reasonable period of time. Common examples of SPM are dust, smoke, fly ash, soot (unburnt carbon particles) etc. Besides, asbestos dust, cement dust, pollen grains, stone dust, dust released in threading, radioactive rays etc. are the common pollutants.
Sources of Air Pollution
There are two main sources of air pollution:
(i) Volcanic eruptions release toxic gases, ash, and heat in enormous quantities which pollute the air.
(ii) Forest fires release harmful gases,
(iii) Electric storms and solar flars produce harmful chemicals in the environment.
(iv) Decay of organic matter produces toxic ammonia gas which pollutes air.
(v) Decay of dead animals and plant bodies in marshy places produces marsh gas (methane,\[C{{H}_{4}}\]) which is natural gas pollutant.
(vi) Pollen grains, spores, cysts, bacteria are also natural air pollutants.
(i) Man has been polluting the air ever since he started using fire.
(ii) Urbanization, industrialization and invention of automobiles as means of transport, all these have speeded up the air pollution.
(iii) Burning of fuels in domestic purpose, mining activities, thermal power plants, nuclear explosions, explosives used in wars, fireworks on festivals are also contributing to air pollution.
(iv) Major harm is being done by burning of fossil fuels (coal, petroleum, natural gas) in automobiles, industries, and thermal power plants.
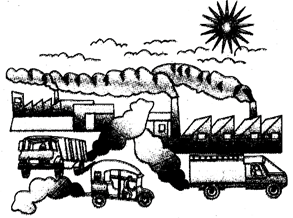
Fig. 3.1: Major sources of air pollution

Fig. 3.2: Major sources of air pollution
Harmful Effects of Air Pollution
Air is required for breathing by humans as well as other land and aquatic animals and plants. Air pollution can thus effect human health as well as human wealth (domestic animals, crop plants, cultural assets etc.) Some common effects of air pollution are:
Human blood has haemoglobin (Hb), a respiratory pigment, which has high affinity for oxygen. Haemoglobin carries oxygen in the form of oxyhaemoglobin from lungs to body tissues.
Carbon monoxide also has 200 times more affinity for haemoglobin than oxygen. When the polluted air containing large amounts of carbon monoxide is inhaled, the CO then combines with haemoglobin to form a stable poisonous compound called carboxyhaemoglobin (Hb. Co).
This drastically reduces the availability of oxygen to the body tissues and leads to suffocation and finally death.
These oxides of sulphur and nitrogen, react with water in the atmosphere to form \[{{H}_{2}}S{{O}_{4}}\]and \[HN{{O}_{3}}\]respectively.
\[2S{{O}_{2}}+{{O}_{2}}\] \[2S{{O}_{3}}\](Sulphur trioxide)
\[S{{O}_{3}}+{{H}_{2}}O\] \[{{H}_{2}}S{{O}_{4}}\](Sulphuric acid)
\[2NO+3[O]\xrightarrow[{}]{{}}{{N}_{2}}{{O}_{5}}\](Nitrogen pentaoxide)
\[{{N}_{2}}{{O}_{5}}+{{H}_{2}}O\xrightarrow[{}]{{}}2HN{{O}_{3}}\](Nitric acid)
These acids are dissolved in water falling down in the form of rain drops. This is called acid rain. When the pH of rain water is less than 5.7, it is called acid rain.
Effects of Acid Rain
(i) Acid rains inhibits \[C{{O}_{2}}\]fixation, photorespiration and productivity of crops.
(ii) Acid rain increases the acidity of soil as well as water bodies, thereby affecting land and aquatic life forms.
(iii) Acid rain also corrodes painted surfaces, metals, slate, stones, and marbles. Our heritage monuments, buildings and statues are threatened by the corrosive action of acid rain. For instance Taj Mahal of Agra is facing such threat.
(iv) It cause irritation in eyes and skin of human beings.
Acid rain problem is increased tremendously by industrialization. Burning of fossil fuel for energy production has been the main cause of acid rain. It has now become a global problem and is needed to be controlled as soon as possible.
Burning of petrol in automobiles releases toxic lead compounds through exhaust pipes of motor vehicles. Continuous inhalation of these compounds causes anaemia, brain damage, convulsions and even death.
Many fungicides and pesticides particles are also suspended in air. They cause severe nerve damage and death.
Measures to Control Air Pollution
(i) Industrial installation should not be allowed to establish in the cities.
(ii) Smoke chimneys of factories should be tall enough.
(iii) Fuel refining policy must be changed so that the emission level could be reduced.
(iv) Automobiles should be made more fuel efficient and less toxic for environment.
(v) Sulphur and lead free fuel should be used for motor vehicles.
(vi) To remove the particulate matter in the smoke, it should be filtered before releasing it into the air.
Large scale planting of trees wherever possible and specially along road sides, reduces air pollution.
Lichens: Natural Indicators of Air Pollution
Lichens are the combination or association of two type of organisms namely algae and fringe. They occur on rocks and tree barks as thin greenish or greyish crust in humid climates.
Lichens are found to be very sensitive to the levels of air polluting gases like sulphur dioxide. Lichens start disappearing in the polluted areas due to which they are called as natural indicator of air pollution.
Water is an inexhaustible natural resource. It is one of the basic necessities of life.
Water persists as liquid above\[0{}^\circ C\]and below\[100{}^\circ C\]. We need water for various activities such as drinking, cooking of food, bathing and washing. It is also needed for irrigation of crops in agriculture, as an essential requirements in industries, fishery, and for navigation.
Water occurs on the earth’s surface in different forms at different places. Rivers, streams lakes, oceans, ponds, pools, polar ice caps, glaciers etc., collectively form the hydrosphere 71% of Earth is covered by oceans which contain 97.5% of the total water. Land contains 2.5% of the total water. A major part (1.9% of the total) of water occurs as ice caps and glaciers. The remaining (0.6% of the total) which supports vole life is called fresh water because it has salt content less than 0.5%. More than 90% of this fresh water is found underground as ground water. Remaining 10% fresh water occurs as surface water (ponds, lakes, dams, rivers), soil water or as water vapours present in the atmosphere. Some quantity of water occurs in the form of body fluids of organisms.
Water is an important part of the living organisms as it performs the following functions for the survival of the living organisms.
Ground Water
During rain fall, some water is lost as run off. This water enters the rivers and reaches seas and some of its gets lost through evaporation. The remaining water percolates into the ground through the pore spaces of the soil particles under the influence of gravity and is termed as ground water. Thus, rainfall is the major source of ground water.
Very minute pore spaces serve as capillaries, allowing the water to move against the pull of gravity. Water moving like this is called capillary water. Some Welter forms an extremely thin, tightly held film around the soil particles. It is termed as hygroscopic water. A small portion of soil water is chemically bound with soil materials and termed as combined water. The downward moving water, called gravitational water, reaches the underground zone called water table, if enough rain falls. Only capillary water is available for plants.
Rain-water Harvesting
Rain-water harvesting is a technique used to capture and store rain water by making special water harvesting structures such as dug-out wells, check dams, percolation pits and lagoons. Rain-water harvesting can be beneficial for all, particularly in the area of scanty rainfall.
The water which rains down cannot percolate through hard end or rocky soil and flows away rapidly to streams, rivers and sea without replenishing the underground water tables.
Underground water table is receding deeper and deeper around the world, due to withdrawal of huge quantity of fresh water continuously. The failure of a centralized distribution system and the depletion of underground water table has forced Indian population to adopt rainwater harvesting and ground water recharge projects.
In the dry areas of the world, rain-water harvesting is an ancient practice. In different parts of Gujarat and Rajasthan, people collect water whatever they can, the quantity of water that is deposited by rain, in large storage bodies, tanks or vessels. This water serves them most of the year.
In another method of rain-water harvesting, rooftops of big buildings properly cleaned are used to collect rain water which is flown through pipes to large underground storage tanks which are sealed of from all sides except for a small opening used for with drawl and cleaning purpose. In cities e.g. Dwarika, Gujarat, people use this collected water for drinking and cooking purpose for most of the year.
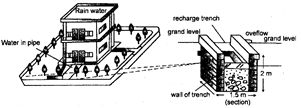
Fig. 3.3: Technique of water harvesting and groundwater recharging
We can improve the ground water holding capacity of earth's crust by using the following methods:
(i) Collection of water deposited over rooftops, open areas, and slopes by erecting obstructions to check the flow.
(ii) Directing this water to underground water table through bore wells and deep shafts drilled in the soil for the purpose so that collected water goes to underground deposits rapidly.
Advantage of rain-water harvesting
The following are the advantages of rain-water harvesting:
(i) It reduces the loss of rain water.
(ii) It maintains a supply of water during the dry months of the year.
(iii) It helps to raise the water table.
(iv) It is helpful in controlling floods.
(v) It checks soil erosion.
(vi) It helps to raise the soil moisture content.
Conservation and Management of Water Resources
In our country, we often face water scarcity. The ground water level goes down in the summer seasons. As a result, during summer seasons the municipal water supply is restricted, our wells and hand pumps become dry. Such frequent situations of water crisis have forced environmental scientists to seek various means of water conservation. They have suggested the following measures for avoiding misuse and wastage of potable water:
Water Pollution
Water pollution may be defined as a change in physical, chemical and biological properties of water by the addition of undesirable substances or the removal of desirable substances from water bodies that adversely affects the aquatic life and makes water less fit or unfit for use.
In other words, the contamination of fresh water due to addition of harmful substance such as foreign organic, inorganic, biological or radioactive substances, making it unfit for use is called water pollution
Water pollution is one of the most serious environmental problems of the world. In India most of our fresh water sources are polluted due to addition of sewage and industrial wastes
Types of Water Pollutants: The substances that pollute the water are called water pollutants These can be classified into three categories:
However, some pollution also occurs, naturally. Soil particles enter water through soil erosion; minerals dissolve in water from rocks and soil; animal wastes and dead fallen leaves (litter) pollute the water sources.
Sources of Water Pollution
The water is polluted by pollutants released by following sources:
7. Thermal Pollution: Hot water released from factories and power generator plants cause thermal pollution of water. The temperature of water in rivers is also affected when water is released from dams. The water inside the deep reservoir would be colder than the water at the surface which gets heated by the sun.
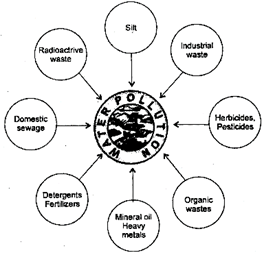
Fig. 3.4: Sources of water pollution
Point and Non-Point Sources of Water Pollution
(a) Point Sources: These sources have a specific location for the discharge of water pollutants directly into water bodies. Therefore, these are located near the water bodies, e.g. petroleum refineries, factories, power plants, oil wells etc. It is always easy to treat the pollutants coming out of point sources before their discharge into water bodies.
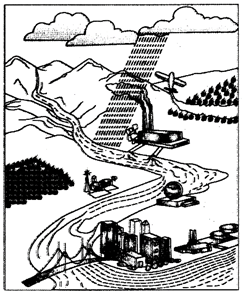
Fig. 3.5: Water may get polluted in many ways
(b) Non-Point Sources: These sources are scattered and do not have any specific location for discharging pollutants into particular water bodies. Examples of non-point sources are run- off from lawn and gardens, fields, roads and streets, construction sites, etc. Unlike point sources these sources are difficult to monitor and treat to remove the pollutants.
Harmful Effects of Water Pollution
Submerged aquatic plants die due to shading. Along with dead algae, the dead submerged plants increase organic loading of water. Decomposition of organic matter consumes dissolved oxygen.
Animals get killed, due to lack of dissolved oxygen. Blue-green algae (EGA) increase in number.
They produce toxins harmful to surviving animals.
Biological magnification of chemicals affects the organisms at every level of the food chain, and maximum at the top level of the food chain.
Soil: The soil (L. Soleus = earthy material in which plants grow) is the uppermost layer of Earth's crust mixed with organic matter. Good soil is made up of mineral particles, humus (organic matter), water, air and microorganisms. The minerals found in the soil layer supply variety of nutrients to life forms. But the soil minerals will not be available to the organism if the minerals are bound up in huge rocks.
Formation of Soil:
Formation of soil involves following two processes: weathering and paedogenesis.
Weathering: It is the breaking down of rock surface into small particles. In the parent rocks, it might have occured for thousands and millions of years. During weathering, the rocks at or near the surface of the earth are broken down by various factors:
(i) Physical Weathering: Various climatic factors such as temperature, rain, water, wind, snowfall, glaciers and running water contribute to physical weathering. High temperature and water cause corrosive humidity and bring about unequal expansion and contraction of rocks, facilitating their break down. Water gets into the cracks in the rocks formed due to uneven heating of different parts of the rocks by the sun, on freezing the water expands in rocks cervices and break the rocks. Flowing water wears away even hard rocks, over long periods of time. Fast flowing water generally carries various-sized particles of rocks downstream on the way, these moving rock particles rub against other rocks. The resultant abrasion forms still smaller particles.
The water takes these particles of rocks and deposits them down its path. In this way soil is found in places far away from its parent rock.
Strong wind influences the formation of soil by continuously rubbing against rocks and eroding them. These also carry sand from one place to other.
(ii) Chemical Weathering: It involves a number of chemical processes such as hydrolysis, hydration, oxidation and reduction. For instance, complex compounds present in the rocks are broken down by the action of carbonic acid present in water or by acidic substances derived from the decomposition of organic matter in soil. The end products of chemical weathering are hydrated oxides, silica, inorganic salts etc.
(iii) Biological Weathering: Living organisms cause biological weathering. Lichens growing on rock surface are able to extract minerals from the same. This creates small crevices at places where a thin layer of soil builds up. Bryophytes (mosses) grow over these crevices. They causes deepening of the crevices and result in buildup of more soil inside them. Deeper crevices form cracks. The cracks become bigger when the roots of short lived herbs, pass into them. With the passage of time roots of bigger plants pass into cracks. Cracks widen and cause slow fragmentation and later pulverisation of rocks.
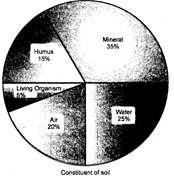
Fig. 4.1
(b) Paedogenesis (Soil Development): It involves stepwise degradation of organic materials by bacteria and fungi (decomposers) and subsequent humiliation and mineralization. Dead remains of animals and plants are called detritus. The process of decomposition involves stepwise degradation of detritus. This process is vital in any ecosystem because it involves release of the nutrients from the dead organic matter. Without this process, all the nutrients would remain locked in the dead remains of animals and plants and will not be available for of living matter.
Humification, Humus and Mineralization Humification is the process by which simplified detritus (partially decomposed organic matter) gets converted into dark coloured amorphous substance called humus in the soil. Humus is the reservoir of nutrients. Humus makes the soil porous, the by, increasing its air and water- holding capacity. The richness of nutrients promotes plant growth. Being black, it absorbs heat to warm up the soil.
Mineralization is the process which results in the release of inorganic substances such as\[C{{O}_{2}},{{H}_{2}}O\]and nutrients (e.g.\[C{{a}^{++}},M{{g}^{++}},N{{H}_{4}}{{,}^{+}},{{K}^{+}}\]etc.) in the soil.
Humus binds the weathered rock particles into aggregate called crumbs. The latter absorb water and hold air in spaces. Nematodes, earth worms, centipedes, millipedes, mites and ants consume organic matter and add excretory nitrogen to it. These are called Detritivores.
Soil Profile
The vertical section of the mature soil upto the depth of 2 meters or upto the parent material to show the different layers of soil in an undistributed state is called soil profile. Soil profile shows four distinct layers called horizons. Horizon A is the top soil. It is dark in colour and of a looser texture than the underlying horizon B. Detritus collects on the surface of this horizon forming the litter.
Below the litter is the humus. The rest of horizon is rich in organic and mineral contents. The horizon B has soil particles smaller and usually more compacted than in the horizon C. Minerals brought by rain water from the upper horizon accumulate in this zone. The horizon C consists of the weathered material derived from the intact parent rocks.
The parent rock forms the horizon D.
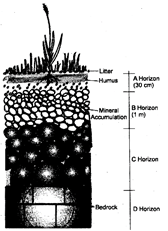
Fig. 4.2
Composition of Soil
Soil particles differ in their size, look and texture. Mainly four types of soil particles are commonly found in top soil. These are:
(i) Gravel: These are large sized particles having a size of greater than 2mm in diameter.
These can be easily picked up by hands.
(ii) Sand Particles: They are coarse to touch and have particle size ranging from 0.05 mm to 2.00 mm. They can be seen easily by naked eye.
(iii) Silt Particles: These are still smaller in size ranging between 0.005 mm to 0.05 mm in diameter.
(iv) Clay Particles: These are the smallest soil particles having size less than 0.005 mm.
Types of Soil
Depending upon the presence of relative amounts of soil particles, soils are classified into following three types:
(i) Sandy Soil: It contains very large proportion of sand particles and very small quantity of silt and clay. It is found in deserts area. It cannot hold much water.
(ii) Clay Soil: It contains large proportion of clay and small amounts of humus and silt. It is compact and can hold water, but cannot trap air.
Loamy Soil: This soil contains relatively larger quantities of clay, silt, sand particles and humus. In fact, it contains about one part clay, two parts silt and two parts sand. This type of soil has good water holding capacity and is porous, to allow aeration of roots.
Major Types of Soil in India
The major types of soils found in India and their composition are presented in following table:
Table 4.3: Major Soil Type of India
|
Sr. No. |
Types of Soil |
Composition |
|
1. |
Black Soil |
Main constituent is clay |
|
2. |
Red Soil |
Sandy loam soil |
|
3. |
Desert soil |
Sandy soil, Poor in organic carbon |
|
4. |
Mountain soil |
Gravel and sandy soil |
|
5. |
Alluvial soil |
Loamy, clay soil |
|
6. |
Laterite soil |
Porous clay soil rich in iron and aluminium hydroxide |
Do You Know
Soil Pollution
Removal of useful components from the soil and addition of harmful or toxic substances which adversely affect the fertility of the soil and kill the diversity of organisms living in it is called soil pollution.
Sources of Soil Pollution
The main sources of soil pollution are as follow:
Effect of Soil Pollution
(i) Solid wastes make the surrounding dirty and emit the foul smell. Due to decomposition of wastes, foul smell spreads by wind to surrounding area to make the life miserable.
(ii) Solid waste may also block the drains which then become the breeding sites of mosquitoes which are the carrier of diseases like malaria and dengue.
(iii) Excessive amount of Fertilizers and pesticides affects the soil fertility and subsequently reduces the crop yield. The soil, thus, may become acidic or alkaline.
(iv) Soil pollution by chemicals, due to dumping of industrial wastes or acid rain, reduces the fertility of soil leading to reduction of crop yields.
(v) Harmful non-biodegradable chemicals can enter the food chains, bio magnify and affect various biotic components including man.
Soil Erosion
The removal and transportation of the top fertile layer of soil from its original position to another place, under the effect of fast running rain water and strong winds, is called soil erosion.
The top layer of soil is rich in humus and very fertile. It provides space for microorganisms and anchorage to plants and is also a source of nutrients and water to the plants.
Soil erosion normally occurs in areas without plant cover (i.e. bare lands). It is because the bare top soil is loose and thus can be easily carried away by erosion factors (i.e. strong wind and fast running rain water).
Causes of Soil Erosion
Effects of Soil Erosion
Prevention of Soil Erosion
Soil erosion can be prevented by providing plant cover to soil. The roots of plants and grasses hold the soil firmly around them. They increase water percolation during rains and decrease run off. Vegetation cover also reduces direct pounding of soil by rain drops. Some important methods to control soil erosion are as follow:
(i) Vegetation cover: Soil should not be left uncovered. Sowing grasses on barren soil or planting of xerophytes will bind the loose soil. The roots of grasses and xerophytes plants hold the soil in place. Vegetation cover also helps in percolating water into deeper layer of soil.
(ii) Contour Bonding: Small bunds are raised on the edges of fields to prevent loss of top soil through wind or water.
(iii) Controlled Grazing: Grazing should be limited to certain reasons and for limited number of animals.
(iv) Wind breaks: Rows of trees and shrubs are planted at right angles to the prevailing wind flow, to check soil erosion by the wind.
(v) Terracing: Slope is divided into a number of flat fields for slowing down the flow of water.
(vi) Conservation tillage: Instead of conventional tillage, reduced or no tillage can be practiced. It prevents soil erosion.
Observation and Conclusion: We observe that in the first set of experiment, more soil will be carried out of tray B. than tray A. In second set of experiment, when more water is poured on the tray and that too from a height, the flowing water further loosen the soil. More soil will be loosened in tray B without plants and will be carried out with water than from tray A having Plants. It is so because in tray A roots of plant seedlings play important role in preventing soil Boston by binding the soil.
Both biotic and abiotic components of the biosphere constantly interact with each other to form a dynamic, but stable system. These interactions include transfer of matter and energy between the two components of the biosphere.
About 30-40 elements are required for the proper growth and development of all the living organisms in nature. These elements tend to circulate in the biosphere from environment to organisms and from organisms back to environment. Some of these elements are-carbon, nitrogen, hydrogen, oxygen, phosphorus, sulphur, calcium etc. The circulation of these elements takes place in the form of a circular path known as biogeochemical cycles. 'Bio’ is referred to living organisms where as "geo" is referred to 'soil rocks, air and water of earth. These cycles involve both biotic and abiotic components such as biological organisms and geological environment and a series of chemical reactions, hence they are termed as 'Biogeochemical Cycles. These are also known as Inorganic-organic cycles or nutrient cycles. Each bio geological cycle is comprised of two types of pools:
From the view point of the ecosphere as a whole, biogeochemical cycles are of two types:
Nutrient fueling, in Ecosystem
Nutrient cycling involves the following three aspects:
(i) Input of nutrients: In this, an ecosystem receives the nutrients from external sources and stores them for their reutilization in the biological processes for the growth and development of livings.
(ii) Output of nutrients: In this, nutrients also move out of an ecosystem and many of them become input to another ecosystem.
(iii) Internal Nutrient Cycling: Soil is the reservoir of most nutrients. They are continuously regenerated and stored in soil in form available to plants. Regeneration of nutrients is done by bacteria, fungi, actinomycetes (decomposers) by the process of decomposition of detritus. These nutrients are stored in soil for their reutilization. Plants absorb huge amount of nutrients from the soil.
The process of nutrients regeneration and absorption occurs simultaneously due to which a dynamic state of nutrients is maintained in the soil.
Transfer of nutrients from the soil by the plants through absorption is known as uptake.
During growth, absorbed nutrients are metabolically incorporated in plants. Litter fall of vegetation, animal remains and faucal matter (above ground detritus) and dead plant roots (below ground detritus) are acted upon by decomposers to bring back the nutrients to the soil- regeneration of nutrients).
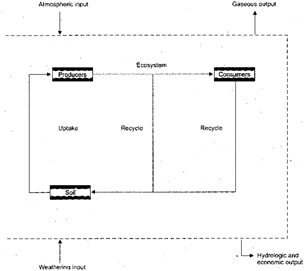
Fig. 5.1: A generalised model of nutrient cycling in an ecosystem
When the uptake of nutrient is more than the amount of nutrient recycled (e.g. a forest where young plants are abundant), a part of the uptake is retained in the standing crop. This retention of nutrients in the standing crop results in the increase in nutrients content of the ecosystem.
Retention = Uptake - Recycle
Rates of nutrient uptake, recycle and retention vary greatly in different ecosystems. The amount of various nutrients/weight of biomass or soil can be determined by a number of chemical methods. We can compute the nutrient budget of the ecosystem by determining changes in the nutrient concentrations and the biomass with time.
Water of Hydrological Cycle
The process in which water evaporates and falls on the land as rain and later flows back into the sea via rivers is known as water cycle. The water cycle actually includes two overlapping cycles-the larger global cycle and smaller water cycle.
(A) Larger Global water cycle: Water evaporates from the hydrosphere i.e. oceans, rivers, lakes, seas etc., with sun's heat and form water vapours in the atmosphere. Condensation of the water vapours results in the formation of clouds. The clouds are blown over the land, where they are cooled enough to drop the water as rain, snow, hail and sleet. Rain may fall directly to the oceans also. Some water from rain and melting snow soaks into the ground, and some flows in rivers and returns directly to seas. The ground water comes to surface again by springs and pumps. It is used in agriculture, industries and domestic purposes. This water finally evaporates into the atmosphere. Water also evaporates from the snow, reservoirs and ground water. Vapours, added by evaporation to the atmosphere, again form clouds. This completes the global water cycle.
(B) Biological or Smaller Water Cycle: In this cycle underground water acts as a starting point. Aquatic organisms take water from their surroundings. The plants may take water from these water bodies and also from the soil while the animals may take water from the plants which they eat, or consume water from different water bodies, or the gravitational water directly.
After the death and decay of these organisms, the water is again made available to plants.
Animals release the water into air by respiration in the form of vapours or to soil as fluid excretion. The plants lose most of the water by the process of transpiration. Water vapours thus formed enter the global water cycle and form clouds. Rain adds water to the soil and reservoirs.
From this water is again made available to plants. So there is exchange of water between the global water cycle and biological water cycle.
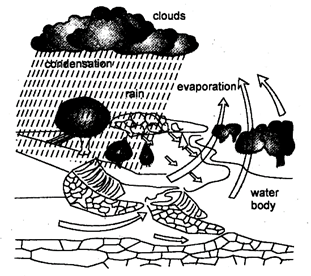
Fig.5.2: Water cycle
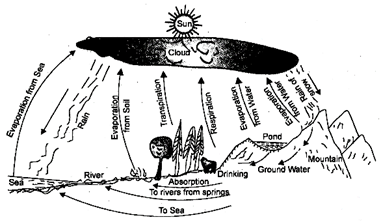
Fig. 5.3 Water cycle in Nature
Nitrogen Cycle
Nitrogen is an essential constituent of protoplasm. Nitrogen is the component of amino acids, proteins, enzymes, nucleotides and nucleic acids. Like all other major and minor elements, it flows in nature in cyclic manner. Nitrogen is picked up as inorganic compounds and is changed into organic form by plants and some prokaryotes. Though atmosphere contains 78.62% of nitrogen in gaseous state, yet plants cannot use it directly. Plants can use nitrogen either in inorganic forms as ammonia, nitrites and nitrates or in organic form such as urea. Animals get nitrogen from plants only. So nitrogen is present both in inorganic (as \[{{N}_{2}},N{{H}_{3}},{{N}_{2}}O,NO,N{{O}_{2}},N{{O}_{{{2}^{-}}}}\]and \[N{{O}_{{{3}^{-}}}}\]) and organic form. So the nitrogen reservoir is atmosphere. Nitrogen cycle is very complex gaseous type of cycle and is perfect cycle. The nitrogen cycle can be conveniently discussed under following heads:
(a) Biological Nitrogen Fixation: It involves the transformation of atmospheric \[{{N}_{2}}\]into nitrites and nitrates by living organisms, such as bacteria and blue-green algae.
Rhizobium is the common symbiotic bacterium that occurs in the nodules of legume roots.
After their death, the proteins and other nitrogen compounds are decomposed to form ammonia and nitrates which become available to plants for assimilation.
(b) Atmospheric Nitrogen Fixation: In the presence of lightning, electric storm and high energy ultraviolet rays, small amounts of nitrogen and oxygen of the air react to form nitrogen oxides. It dissolves in rain water and pass down to soil and water bodies as nitrites and nitrates.
(c) Industrial Nitrogen Fixation: Nitrogen is made to combine with hydrogen under high temperature and pressure to form ammonia. Ammonia is often changed further to urea because the latter is less toxic.
II. Nitrogen Assimilation: It is carried out by plants. Plants pick up nitrates or ammonium ions from soil. Nitrate is first changed into ammonium state. Ammonium ions combine with organic acid to produce amino acids. Amino acids give rise to protein and nucleotides. Nucleotides produce nucleic acids.
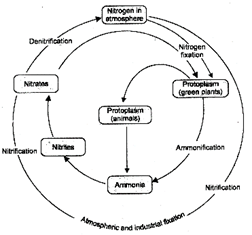
Fig. 5.4: Nitrogen cycle
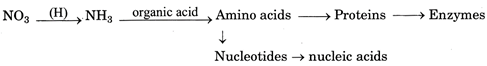
Animals receive organic nitrogen directly or indirectly from plants. Ingested proteins and other complex compounds are first broken down to soluble state of amino acids, nucleotides etc.
They are absorbed and converted into parts of animal protoplasm.

Fig. 5.5: Nitrogen cycle
III. Ammonification: Proteins and other complex nitrogenous compounds of organic remains are acted upon by decomposers. They are first hydrolysed to form amino acids which –are deaminated to release ammonia and form organic acids. The phenomenon is called ammonification.
Proteins—amino acids—Organic acids + Ammonia
Ammonia undergoes nitrification with the help of nitrifying bacteria. Nitrosomonas oxidise ammonia to nitrite while Nitrobacter oxidises it further to nitrate. Nitrate is reabsorbed by plants for assimilation.
\[NO_{3}^{-}\xrightarrow[{}]{{}}N{{O}_{2}}^{-}\](Nitrate)
\[NO_{2}^{-}\xrightarrow[{}]{{}}N{{O}^{-}}\](Nitrous oxide)
\[NO\xrightarrow[{}]{{}}{{N}_{2}}O\](Nitric acid)
\[{{N}_{2}}O\xrightarrow[{}]{{}}{{N}_{2}}\](Nitrogen)
Table 5.6: Organisms involved in Nitrogen Fixation
|
Name of microorganisms |
Role played in nitrogen cycle |
|
1. (a) Rhizobium bacteria (in root nodules) |
|
|
(b) Azotobactor bacteria (in soil) (c) Blue-green algae |
|
|
2. (a) Putrefying bacteria (b) Fungi |
|
|
3. Nitrifying bacteria (a) Nitrobacter (b) Nitrosamines |
|
|
4. De-nitrifying Bacteria (Pseudomonas) |
Conversion of nitrate salts into free nitrogen gas |
Oxygen Cycle
Oxygen forms about 20.84% of the atmosphere. Organisms respire aerobically in the presence of oxygen. It is found as a component of oxidised salts \[C{{O}_{2}}\]and \[{{H}_{2}}O\].
Oxygen enters the living world through respiration; it oxidises the food material and produces energy and \[C{{O}_{2}}\].
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{O}_{2}}\xrightarrow[{}]{{}}6C{{O}_{2}}+6{{H}_{2}}O+\]Energy
The carbon dioxide is utilised by the plants to produce food materials during the process of photosynthesis.
\[\underset{(Carbon\,dixoide)}{\mathop{6C{{O}_{2}}}}\,+\underset{(water)}{\mathop{12{{H}_{2}}O}}\,\xrightarrow[Chlorophyll]{Light}\underset{(glucose)}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{(water)}{\mathop{6{{H}_{2}}O}}\,+\underset{(oxygen)}{\mathop{6{{O}_{2}}}}\,\]
The consequent free oxygen may now be used again in respiration or it may return to environment as molecular oxygen of atmosphere thus, completing the biogeochemical cycle.
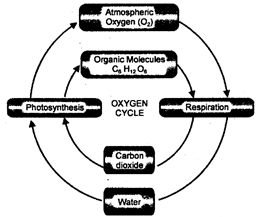
Fig. 5.7: Oxygen cycle in nature
The process of photosynthesis and respiration maintain the concentration of oxygen in the air and water. Oxygen is also released as a part of\[C{{O}_{2}}\]by decomposition of dead organic matter Some\[{{O}_{2}}\]is added to the air as \[C{{O}_{2}},{{H}_{2}}O,S{{O}_{2}},\]and during burning of fules (coal, wood petroleum etc.) Some oxides (e.g. nitrate and nitrites) are formed by microbial oxidation process.
These oxides produce \[{{O}_{2}}\]when reduced by chemical and biological process. \[{{O}_{2}}\]is temporarily withdrawn from the circulation, when it combines with soil minerals to form these oxides.
Some part of the atmospheric oxygen that react at the high levels of the atmosphere is converted to ozone \[({{O}_{3}})\]by high energy UV-radiation. The ozone forms the protective ozone layer of the atmosphere.
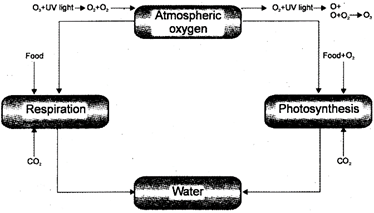
Fig. 5.8
As the oxygen concentration in the atmosphere remains constant at 21% it is likely that natural degradation of ozone must occur to maintain the ozone: oxygen equilibrium.
Carbon Cycle
Carbon is present in the following forms on the earth:
(a) In the elemental forms as diamond and graphite.
(b) As carbon dioxide\[(C{{O}_{2}})\]in the atmosphere.\[C{{O}_{2}}\]is the major source of carbon for the living world. The atmosphere contains 0.03 to 0.04% of\[C{{O}_{2}}\].
(c) As carbonate and hydrogen carbonate salts in various minerals. The endoskeletons and exoskeletons of various animals are also formed of carbonate salts.
(d) As proteins, carbohydrates, fats, nucleic acids (DNA and RNA) and vitamin in all the life forms.
Steps of Carbon Cycle
In turn consume herbivores. Thus, all animals, directly or indirectly obtain their supply of carbon from green plants:
Producers\[\to \]Herbivores\[\to \]Carnivores (green plants)
3 Respiration: Carbon is released back as carbon dioxide into the environment by plants and animals through respiration. During respiration, oxygen may or may not be used to convert glucose back into\[C{{O}_{2}}\].
4. Decay: Plants and animals also release \[C{{O}_{2}}\]during decay.
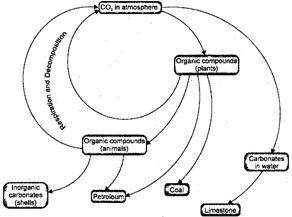
Fig. 5.9: Carbon cycle in nature
In fact, the percentage of \[C{{O}_{2}}\]in the atmosphere in said to have doubled sine the industrial revolution when human beings started burning fossil fuels on very large scale.
Green house is a glass enclosure which is used for growing tropical plants in colder climates even in winters. The glass walls of greenhouse allow sun rays to pass into the interior right upto earth's surface. However, reflected back infra-red rays are not allowed to escape by the glass walls as well as\[C{{O}_{2}}\], gas and water vapours present in the air, inside the glass house. This make greenhouse air warmer than the outside air.
The atmosphere of the earth works like glass walls of the green house. It allows solar radiations to pass through it to strike the earth surface. However, it prevents the long wave infra-red radiation to escape into the space. Gases such as carbon dioxide\[(C{{O}_{2}})\], methane\[(C{{H}_{4}})\], chlorofluorocarbons (CFCs), oxides of nitrogen\[({{N}_{2}}O)\], etc. are called greenhouses gases. Out of these, \[C{{O}_{2}}\]is the most important greenhouse gas. These gases are important in keeping the earth warm. The mean average temperature of the earth is around\[15{}^\circ C\]. In the absence of greenhouse gases, it may drop to\[-20{}^\circ C\], killing all forms of life on the earth.
Human is adding huge amounts of \[C{{O}_{2}}\]and \[C{{H}_{4}}\]to the atmosphere by burning of fossil fuels in homes, automobiles, industries, burning associated with agricultural practices; as well as by deforestation. Methane is also added to atmosphere by the decay of vegetable matter in marshes, paddy fields and biogas plants. These greenhouse gases (mainly \[C{{O}_{2}}\]and\[C{{H}_{4}}\]) trap the heat (infrared radiation) reflected back by the earth. This heat up the atmosphere. This phenomenon is known as global warming which occur due to greenhouse effect.
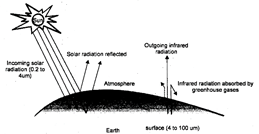
Fig. 5.10: Greenhouse effect
Effect of Global Warming
(i) Increase in temperature of earth's environment even by\[1{}^\circ C\]can lead to melting of ice present on the poles of the earth. This will result in rise in the sea level. Thus, coastal areas will be flooded or might even be submerged.
(ii) Increase in temperature of earth due to greenhouse effect will result in change in weather and precipitation patterns.
(iii) Due to increasing temperature higher incidence of disease will occur.
Elemental oxygen is normally found in the form of a diatomic molecule. However in the upper part of atmosphere, oxygen is found in the form of Ozone, having three oxygen atoms Ozone is found in stratosphere also called ozonosphere. It is the region in the upper atmosphere between about 18-50 km, in which there are appreciable concentration of\[{{O}_{3}}\]. Ozone exists as thick blanket called Ozone Shield. If absorb harmful UV-radiation and prevents them from reaching the earth surface.
Electrical sparks and ultraviolet radiations can cause ordinary oxygen to form ozone.
\[{{O}_{2}}+O\]
\[{{O}_{3}}\xrightarrow[{}]{UV\,radiation}O+{{O}_{2}}\]
Ozone is considered as a pollutant at ground level, but the ozone layer of the upper atmosphere protects life on earth from Sun's harmful ultraviolet radiations which can cause many disease like skin cancer, eye cataracts, genetic disorders and suppressed immune system etc.
Ozone layer depletion:
Certain air pollutants (used as refrigerants now-a-day) particularly Chlorofluorocarbons (CFCs, carbon compounds having both fluorine and chlorine which are very stable and not degraded by any biological process) and chlorofluoro bromine compounds can diffuse into the ozonosphere and destroy ozone. A single atom of chlorine (Cl) released from CFCs, for example, floating about in the upper atmosphere, can destroy hundreds of thousands of molecules of ozone because the chlorine acts as a catalyst and is not itself altered in the process. Hence the ozone layer is stated to be depleting day by day.

Fig. 5.11: Satellite pictures showing the hole in the ozone layer over Antarctica
In the Mid 1980s scientists discovered that a 'hole' developed in the ozonosphere above Antarctica and it was found that there, the ozone layer was thinned by as much as 40-50% from its normal concentration. This severe regional ozone depletion was explained as a natural phenomenon, but simultaneously it was also considered as a result of some air pollutants like CFCs and haloes. These chemical compounds react with the ozone gas present in the atmosphere and decompose it. Concern over increasing global ozone depletion led to international restrictions on the use of CFCs and haloes and to scheduled reduction in their manufacture.
Chapter at a Glance
You need to login to perform this action.
You will be redirected in
3 sec
