Fuels
- Substances that produce heat on combustion are called fuels.
DIFFERENT FORMS OF FUEL
There are different forms of fuels:
- Biomass fuel: The material contained in the bodies of plants and animals is called biomass. When organisms die, their biomass can be used as fuel for domestic purposes.
- Agricultural wastes: Bagasse (sugarcane from which juice has been extracted) is sometimes burned in industrial boilers.
- Wood: We bum wood in traditional chulhas.
- Dung cakes: Animal dung in the form of dried cakes is also burned in many parts of the country for domestic purposes.
- Biogas: It is unadvisable to bum dung cakes directly because animal dung contains vital nutrients should be returned to the soil. As duns cakes bum inefficiently and produce a lot of smoke, their utilisation as fuel leads to a considerable wastage of useful elements and causes air pollution. Instead, animal dung should be converted into biogas, which is a cleaner fuel. The residue, which is rich in nutrients, can then be used as manure.
Animal and plant wastes are easily degraded by anaerobic microorganisms in the presence of water. In this process, gases such as methane, carbon dioxide, hydrogen sulphide are produced.
This mixture of gases is called biogas. It contains about 65 per cent methane, which is an excellent fuel. Biogas can be burned in gas stoves to produce heat. It can also be used for street lighting and for running engines.
- Fossil fuels: Fossil fuels are formed over millions of years by the burial of plant and animal remains.
The most commonly used fossil fuels are: (i) coal (ii) petroleum (iii) natural gas.
(i) Coal
- Coal deposits are the distilled remains of large land plants which flourished in vast shallow swamps about 300 million years ago.
- In India, coal is mainly found in Bihar, Orissa, Madhya Pradesh and West Bengal.
- It consists largely of atoms of carbon, hydrogen, and oxygen, and a small amount of sulphur.
- It comes in three main varieties—lignite, bituminous and anthracite.
- Coal, when heated in the absence of air, gives coke. Coal tar and coal gas can be obtained from coal by a simple process known as destructive distillation.
- Coal tar is a mixture of many carbon compounds. For about a hundred years, it was the chief source of important carbon compounds used in dyes, explosives, paints, synthetic fibre, drugs and pesticides.
- Coal gas is a mixture of hydrogen, methane and carbon monoxide. This mixture is an excellent fuel. It was used for lighting homes, factories and streets.
(ii) Petroleum
- Petroleum is formed by decomposition of microorganisms and dead organic matter. The dead organic remains sinks to the bottom and are gradually covered by sand and clay. Over millions of years, these remains are gradually transformed into hydrocarbons by heat, pressure and catalytic action. These hydrocarbons rise through the permeable rock layers. They form an oil trap when blocked by some impermeable rock layers.
- Petroleum is obtained by drilling a hole through the impervious cap rock.
- Petroleum is a mixture of several hydrocarbons.
- Crude oil is a brown-black liquid.
- Petrol and diesel are mainly used in automobiles and locomotives. Two wheelers, three wheelers and most motor cars run on petrol. Heavier vehicles such as buses, trucks and railway engines run on diesel. Diesel is also used to operate pumps in the fields and to generate electricity on a small scale.
(iii) Natural Gas
- Natural gas readily bums to produce heat. It is also a rich source of hydrogen gas which is needed to manufacture fertilisers. It occurs along with oil. Many oil wells produce natural gas as a by-product. There are, however, some wells that produce only natural gas as a by-product.
- Natural gas mainly contains methane \[(C{{H}_{4}})\] Its great advantage is that it can be used directly for burning purposes in homes and factories.
Other Fuels
- Kerosene: Kerosene is used for domestic purposes like in a pressure or wick stove to obtain heat, for lighting hurricane or petromax lamps for illumination. A special grade of kerosene is also used as aviation fuel in aeroplane jet engines.
- Petroleum gas: It is a mixture of ethane, propane and butane. Its main constituent is butane, which bums readily, releasing a lot of heat. Butane is easily liquefied under pressure. It is supplied in liquid form in cylinders and is commonly known as Liquified Petroleum Gas (LPG).
CLASSIFICATION OF FUELS
Fuels are classified in two ways:
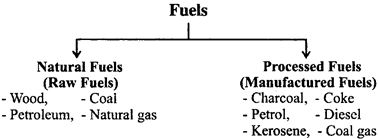
- Fuels are also be classified in other ways:
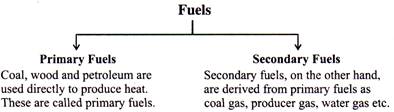
COMBUSTION OF FUELS
Combustion means burning. During combustion, an element or a compound combines with oxygen. It is an exothermic process, i.e., heat is given out in this reaction. Oxygen is essential for burning. In most cases, oxygen is available from the air. In wood-fired chulhas, gaps are left between the logs for the air to enter and facilitate their burning.
Harmful effects of over-combustion of fuels
As our energy requirements continue to grow, we are burning more and more fuels. In doing so, we also add undesirable and dangerous chemicals to the air. For example, coal contains carbon, hydrogen, nitrogen and sulphur. Carbon, in the presence of enough oxygen, produces a harmless and non-toxic gas carbon dioxide. An increased proportion of carbon dioxide in the air could lead to undesirable changes in the climate. However, if the oxygen supply is insufficient, carbon bums incompletely to produce the poisonous gas carbon monoxide. Also, while nitrogen usually escapes as a free element, sulphur present in the coal forms sulphur dioxide, which is an extremely suffocating and corrosive gas.
Burning of petroleum
Petroleum-based fuels also lead to air pollution. When gasoline bums in automobiles, it produces water vapour, carbon dioxide, carbon monoxide and unburnt hydrocarbons. All these materials are released from the high temperature of internal combustion engines. Even nitrogen from the air is oxidised to nitrogen oxides. Except for carbon dioxide and water vapour, all these components are harmful.
ATTRIBUTES OF AN IDEAL FUEL
Some attributes of an ideal fuel are:
(i) High calorific value
(ii) Proper ignition temperature
(iii) Moderate rate of combustion
(iv) Low content of non-volatile material
(v) Absence of poisonous products on combustion
(vi) Ready and plentiful availability
(vii) Low cost
(viii) Ease of storage and transportation.


