Disaccharides
Category : JEE Main & Advanced
The disaccharides yield on hydrolysis two monosaccharides. Those disaccharides which yield two hexoses on hydrolysis have a general formula \[{{C}_{12}}{{H}_{22}}{{O}_{11}}.\] The hexoses obtained on hydrolysis may be same or different.
\[\underset{\text{Sucrose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
Lactose \[\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\] Glucose + Galactose
Maltose \[\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\]Glucose + Glucose
The hydrolysis is done by dilute acids or enzymes. The enzymes which bring hydrolysis of sucrose, lactose and maltose are invertase, lactase and maltase, respectively. Out of the three disaccharides, sucrose (cane-sugar) is the most important as it is an essential constituent of our diet.
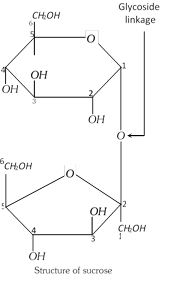
In disaccharides, the two monosaccharides are joined together by glycoside linkage. A glycoside bond is formed when hydroxy group of the hemiacetal carbon of one monosaccharide condenses with a hydroxy group of another monosaccharide giving \[-O-\] bond.
(1) Sucrose; Cane-sugar \[\mathbf{[}{{\mathbf{C}}_{\mathbf{12}}}{{\mathbf{H}}_{\mathbf{22}}}{{\mathbf{O}}_{\mathbf{11}}}\mathbf{]}\] : It is our common table sugar. It is obtained from sugar cane and sugarbeets. It is actually found in all photosynthetic plants.
(i) Properties : It is a colourless, odourless, crystalline compound. It melts at \[185{{186}^{o}}C\]. It is very soluble in water, slightly soluble in alcohol and insoluble in ether. It is dextrorotatory but does not show mutarotation. It is a non-reducing sugar as it does not reduce Tollen’s or Fehling’s reagent. Sucrose, on heating slowly and carefully, melts and then if allowed to cool, it solidifies to pale yellow glassy mass called ‘Barley sugar’. When heated to \[{{200}^{o}}C\], it loses water to form brown amorphous mass called Caramel. On strong heating, it chars to almost pure carbon giving smell of burnt sugar. It is composed of \[\alpha -D-\]glucopyranose unit and a \[\beta -D-\]fructofuranose unit. These units are joined by \[\alpha -\beta -\]glycosidic linkage between \[C-1\] of the glucose unit and \[C-2\] of the fructose unit.
(ii) Uses
(a) As a sweetening agent for various food preparations, jams, syrups sweets, etc.
(b) In the manufacture of sucrose octa-acetate required to denature alcohol, to make paper transparent and to make anhydrous adhesives.
(2) Inversion of cane-sugar : The hydrolysis of sucrose by boiling with a mineral acid or by enzyme invertase, produces a mixture of equal molecules of D-glucose and D-fructose.
\[\underset{\text{Sucrose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}}\underset{\text{(This mixture is laevorotatory)}}{\mathop{\underset{\text{D-Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{D-Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,}}\,\]
Sucrose solution is dextrorotatory. Its specific rotation is \[+{{66.5}^{o}}.\] But on hydrolysis, it becomes laevorotatory. The specific rotation of D-glucose is \[+{{52}^{o}}\] and of D-fructose is \[-{{92}^{o}}.\] Therefore, the net specific rotation of an equimolar mixture of D-glucose and D-fructose is.
\[\frac{+{{52}^{o}}-{{92}^{o}}}{2}=-{{20}^{o}}\]
Thus, in the process of hydrolysis of sucrose, the specific rotation changes from \[+\,\,66.5{}^\circ \] to \[-\,\,20{}^\circ \], i.e., from dextro it becomes laevo and it is said that inversion has taken place. The process of hydrolysis of sucrose is thus termed as inversion of sugar and the hydrolysed mixture having equal molar quantities of D-glucose and D-fructose is called invert sugar. The enzyme that brings the inversion is named as invertase.
Distinction between glucose and sucrose
| Test | Glucose | Sucrose |
| With conc. \[{{H}_{2}}S{{O}_{4}}\] in cold | No effect | Charring occurs and turns black |
| Molisch’s reagent | Violet ring is formed | Violet ring is formed |
| With NaOH | Turns yellow | No effect |
| With Tollen’s Solution | Gives silver mirror | No effect |
| With Fehling’s solution | Gives red precipitate of \[C{{u}_{2}}O\] | No effect |
| On heating with phenyl hydrazine | Gives yellow precipitate of glucosazone | No effect, i.e., does not form osazone |
| Aqueous resorcinol + conc. HCl solution | No effect | Reddish-brown precipitate which dissolves in ethanol. |
You need to login to perform this action.
You will be redirected in
3 sec
