Dicarboxylic Acids
Category : JEE Main & Advanced
The acids containing two carboxylic groups are called dicarboxylic acids.
The saturated dicarboxylic acid are represented by the general formula \[{{C}_{n}}{{H}_{2n}}{{(COOH)}_{2}}\] where n = 0, 1, 2, 3 etc.
\[HO-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-{{(C{{H}_{2}})}_{n}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-OH\] or \[HOOC{{(C{{H}_{2}})}_{n}}COOH\]
According to IUPAC system, the suffix-dioic acid is added to the name of parent alkane, i.e. Alkane dioxic acid.
| Formula | Common name | IUPAC name |
| \[HOOCCOOH\] | Oxalic acid | Ethanedioic acid |
| \[HOOCC{{H}_{2}}COOH\] | Malonic acid | 1-3 Propanedioic acid |
| \[HOOCC{{H}_{2}}C{{H}_{2}}COOH\] | Succinic acid | 1,4-Butanedioic acid |
| \[HOOC{{(C{{H}_{2}})}_{3}}COOH\] | Glutaric acid | 1,5-Pentanedioic acid |
| \[HOOC{{(C{{H}_{2}})}_{4}}COOH\] | Adipic acid | 1,6-Hexanedioic acid |
Oxalic Acid or Ethanedioic Acid
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\ \,\,\,\,\,\,\,}}}\,\] or \[{{(COOH)}_{\mathbf{2}}}\]or \[({{C}_{\mathbf{2}}}{{H}_{\mathbf{2}}}{{O}_{\mathbf{4}}})\]
Oxalic acid is first member of dicarboxylic series.
It occurs as potassium hydrogen oxalate in the wood sorel, rhubarb and other plants of oxalis group and as calcium oxalate in plants of rumex family.
It is found in the form of calcium oxalate in stony deposits in kidneys and bladdar in human body.
Oxalic acid present in tomatoes.
(1) Methods of Preparation
(i) By oxidation of ethylene glycol with acidified potassium dichromate
\[\underset{\text{Glycol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+4[O]\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,\,+2{{H}_{2}}O\]
(ii) By hydrolysis of cyanogen with conc. hydrochloric acid : \[\underset{CN}{\overset{CN}{\mathop{|\,\,\,\,}}}\,+4{{H}_{2}}O\xrightarrow{2(HCl)}\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,}}}\,+2N{{H}_{4}}Cl\]
(iii) By heating sodium or potassium in a current of carbon dioxide at \[{{360}^{o}}C\]
\[2Na+2C{{O}_{2}}\xrightarrow{\text{heat}}\,\underset{\text{Sodium oxalate}}{\mathop{\underset{COONa}{\overset{COONa}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iv) Laboratory preparation
\[\underset{\text{Sucrose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+18[O]\underset{{{V}_{2}}{{O}_{5}}}{\mathop{\xrightarrow{HN{{O}_{3}}}}}\,6\underset{\text{Oxalic acid}}{\mathop{\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+5{{H}_{2}}O\]
(v) Industrial method
\[\underset{\text{Sod}\text{. formate}}{\mathop{2HCOONa}}\,\xrightarrow{360{}^\circ C}\underset{\text{Sod}\text{. oxalate}}{\mathop{\underset{COONa}{\overset{COONa}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+{{H}_{2}}\]
Sodium formate is obtained by passing carbon monoxide over fine powdered of sodium hydroxide.
\[CO+NaOH\underset{8-10atm}{\mathop{\xrightarrow{200{}^\circ C}}}\,HCOONa\].
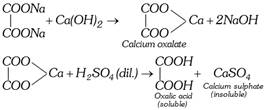
The sodium oxalate thus formed is dissolved in water and calcium hydroxide is added. The precipitate of calcium oxalate is formed which is separated by filtration. It is decomposed with calculated quantity of dilute sulphuric acid.
(2) Physical Properties
(i) It is a colourless crystalline solid. It consists of two molecules of water as water of crystallisation.
(ii) The hydrated form has the melting point \[101.5{}^\circ C\] while the anhydrous form melts at\[190{}^\circ C\].
(iii) It is soluble in water and alcohol but insoluble in ether.
(iv) It is poisonous in nature. It affects the central nervous system.
(3) Chemical Properties
(i) Action of heat : It becomes anhydrous.
\[\underset{\begin{smallmatrix} \text{Hydrated oxalic} \\ \text{ acid}\end{smallmatrix}}{\mathop{{{(COOH)}_{2}}2{{H}_{2}}O}}\,\xrightarrow{100-105{}^\circ C}(\underset{\begin{smallmatrix} \text{Anhydrous} \\ \text{oxalic acid} \end{smallmatrix}}{\mathop{COOH{{)}_{2}}}}\,+2{{H}_{2}}O\]
(a) At \[200{}^\circ C\], \[{{(COOH)}_{2}}\xrightarrow{{}}\underset{\text{Formic acid}}{\mathop{HCOOH}}\,+C{{O}_{2}}\]
On further heating, formic acid also decomposes.
\[HCOOH\to C{{O}_{2}}+{{H}_{2}}\]
(b) Heating with conc. H2SO4
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,\underset{(conc.)}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,CO+C{{O}_{2}}+{{H}_{2}}O\]
(ii) Acidic nature
Salt formation
\[\underset{\text{Oxalic acid}}{\mathop{\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+KOH\to \underset{\text{Acid pot}\text{. oxalate}}{\mathop{\underset{COOK}{\overset{COOK}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{KOH}\underset{\text{Pot}\text{. oxalate}}{\mathop{\underset{COOK}{\overset{COOK}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,+2NaHC{{O}_{3}}\to \underset{\text{Sod}\text{. oxalate}}{\mathop{\underset{COONa}{\overset{COONa}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+2C{{O}_{2}}+2{{H}_{2}}O\]
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,+N{{a}_{2}}C{{O}_{3}}\to \underset{COONa}{\overset{COONa}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,+{{H}_{2}}O+C{{O}_{2}}\]
(iii) Esterification
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{{{C}_{2}}{{H}_{5}}OH}\underset{\begin{smallmatrix} \text{Ethyl hydrogen} \\ \,\,\,\,\,\,\text{oxalate} \end{smallmatrix}}{\mathop{\underset{COOH\,\,\,\,\,\,\,\,}{\overset{COO{{C}_{2}}{{H}_{5}}}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{C}_{2}}{{H}_{5}}OH}\underset{\text{Ethyl oxalate}}{\mathop{\underset{COO{{C}_{2}}{{H}_{5}}}{\overset{COO{{C}_{2}}{{H}_{5}}}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iv) Reaction with \[PC{{l}_{5}}\] :
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,+2PC{{l}_{5}}\to \underset{\begin{smallmatrix} \text{Oxalyl} \\ \text{chloride} \end{smallmatrix}}{\mathop{\underset{COCl}{\overset{COCl}{\mathop{|\,\,\,\,\,\,\,\,\,}}}\,}}\,+2POC{{l}_{3}}+2HCl\]
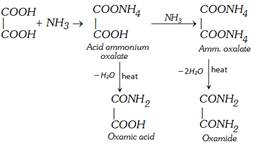
(v) Reaction with ammonia
(vi) Oxidation : When oxalic acid is warmed with acidified \[KMn{{O}_{4}}\].
\[2KMn{{O}_{4}}+3{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+3{{H}_{2}}O+5[O]\]
\[\frac{\left[ \begin{matrix} COOH \\ \overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{COOH}}\, \\ \end{matrix}+[O]\to 2C{{O}_{2}}+{{H}_{2}}O \right]\times 5}{\underset{\begin{smallmatrix} \text{Pot}\text{. permanganate} \\ \,\,\,\,\,\,\,\,\text{(Purple)} \end{smallmatrix}}{\mathop{2KMn{{O}_{4}}}}\,+3{{H}_{2}}S{{O}_{4}}+5\,\underset{\text{Oxalic acid}}{\mathop{\begin{matrix} \underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{COOH}}\, \\ COOH \\ \end{matrix}}}\,\to {{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+10C{{O}_{2}}+8{{H}_{2}}O}\]
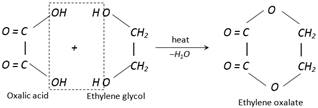
(vii) Reaction with ethylene glycol
(viii) Reduction :
\[\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,+4H\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{Zn}}}\,\underset{\text{Glycolic acid}}{\mathop{\underset{COOH\,\,}{\overset{C{{H}_{2}}OH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+{{H}_{2}}O\]
\[2\underset{COOH}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,}}}\,\underset{6[H]}{\mathop{\xrightarrow{\text{Electrolytic reduction}}}}\,\underset{\text{Glycolic acid}}{\mathop{\underset{COOH\,\,\,}{\overset{C{{H}_{2}}OH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+\underset{\text{Glyoxalic acid}}{\mathop{\underset{CHO\,\,\,\,}{\overset{COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+2{{H}_{2}}O\]
(ix) Reaction with Glycerol : At \[100{}^\circ 110{}^\circ C,\] formic acid is formed. At \[260{}^\circ ,\] allyl alcohol is formed.
(4) Uses : Oxalic acid (Polyprotic acid) is used,
(i) In the manufacture of carbon monoxide, formic acid and allyl alcohol.
(ii) As a laboratory reagent and as a standard substance in volumetric analysis.
(iii) In the form of antimony salt as a mordant in dyeing and calico printing.
(iv) In the manufacture of inks.
(v) For removing ink stains and rust stains and for bleaching straw, wood and leather.
(vi) In the form of ferrous potassium oxalate as developer in photography.
(5) Analytical test
(i) The aqueous solution turns blue litmus red.
(ii) The aqueous solution evolves effervescences with \[NaHC{{O}_{3}}\].
(iii) The neutral solution gives a white precipitate with calcium chloride solution. It is insoluble in acetic acid.
\[\underset{\text{Oxalic acid}}{\mathop{{{H}_{2}}{{C}_{2}}{{O}_{4}}}}\,\xrightarrow{N{{H}_{4}}OH}\underset{\text{Amm}\text{.oxalate}}{\mathop{{{(N{{H}_{4}})}_{2}}{{C}_{2}}{{O}_{4}}}}\,\xrightarrow{CaC{{l}_{2}}}\underset{\text{Calcium oxalate}}{\mathop{Ca{{C}_{2}}{{O}_{4}}}}\,\]
(iv) Oxalic acid decolourises hot potassium permanganate solution having dilute sulphuric acid.
(v) With hot conc. \[{{H}_{2}}S{{O}_{4}}\], it evolves carbon monoxide which burns with blue flame.
Malonic Acid or Propane-1,3-Dioic Acid
\[C{{H}_{2}}\ \ \ \ \ \begin{matrix} COOH \\ COOH \\ \end{matrix}\] or \[C{{H}_{2}}{{(COOH)}_{2}}\] or \[({{C}_{3}}{{H}_{4}}{{O}_{4}})\]
The acid occurs as calcium salt in sugar beet. It was so named because it was first obtained from malic acid (hydroxy succinic acid) by oxidation.
(1) Methods of Preparation : From acetic acid
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\underset{P}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{\text{Chloroacetic acid}}{\mathop{C{{H}_{2}}ClCOOH}}\,\xrightarrow{KCN(Aq.)}\]
![]()
(2) Physical Properties
(i) It is a white crystalline solid.
(ii) It's melting point is \[135{}^\circ C\].
(iii) It is soluble in water and alcohol but sparingly soluble in ether.
(3) Chemical Properties
(i) Action of heat
(a) Heating at \[150{}^\circ C\] :
\[C{{H}_{2}}{{(COOH)}_{2}}\to C{{H}_{3}}COOH+C{{O}_{2}}\]
(b) Heating with \[{{P}_{2}}{{O}_{5}}\]:
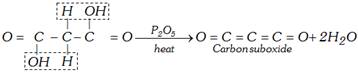
(ii) Reaction with aldehyde : With aldehydes, \[\alpha -\beta \] unsaturated acids are formed.
![]()
\[\underset{\alpha \text{-}\beta \text{ unsaturated acid}}{\mathop{RCH=CHCOOH}}\,+{{H}_{2}}O+C{{O}_{2}}\]
(4) Uses : Its diethyl ester (malonic ester) is a valuable synthetic reagent for preparation of a variety of carboxylic acids.
Succinic Acid or Butane-1,4-Dioic Acid :
\[\underset{C{{H}_{2}}-COOH}{\overset{C{{H}_{2}}-COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\] or \[{{(C{{H}_{2}})}_{2}}{{(COOH)}_{2}}\] or \[({{C}_{4}}{{H}_{6}}{{O}_{4}})\]
It was first obtained by the distillation of yellow fossil, resin, amber and hence its name (Latin, Succinum = amber).
It is also formed in small amount during the fermentation of sugar.
(1) Methods of Preparation
(i) From ethylene
\[\underset{\text{Ethylene}}{\mathop{\underset{C{{H}_{2}}}{\overset{C{{H}_{2}}}{\mathop{|\,|\,\,\,\,\,\,}}}\,}}\,\xrightarrow{B{{r}_{2}}}\underset{\begin{smallmatrix} \text{Ethylene} \\ \text{bromide} \end{smallmatrix}} {\mathop{\underset{C{{H}_{2}}Br}{\overset{C{{H}_{2}}Br}{\mathop{|\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{NaCN}\underset{\begin{smallmatrix} \text{Ethylene} \\ \text{cyanide} \end{smallmatrix}}{\mathop{\underset{C{{H}_{2}}CN}{\overset{C{{H}_{2}}CN} {\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}_{2}}O}/{HCl}\;}\underset{\text{Succinic acid}}{\mathop{\underset{C{{H}_{2}}COOH} {\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}COOH}}\,}}\,}}\,\]
(ii) From maleic acid [catalytic reduction]
\[\underset{CHCOOH}{\overset{CHCOOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,+{{H}_{2}}\underset{\text{heat}}{\mathop{\xrightarrow{Ni}}}\,\underset{C{{H}_{2}}COOH}{\overset{C{{H}_{2}}COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\]
(iii) Reduction of tartaric acid or malic acid
\[\underset{\text{Tartaric acid}}{\mathop{\underset{CHOHCOOH}{\overset{CHOHCOOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{P}{\mathop{\xrightarrow{HI}}}\,\underset{\text{Succinic acid}}{\mathop{\underset{C{{H}_{2}}COOH}{\overset{C{{H}_{2}}COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{P}{\mathop{\xleftarrow{HI}}}\,\underset{\text{Malic acid}}{\mathop{\underset{C{{H}_{2}}COOH\,\,\,\,\,\,}{\overset{CHOHCOOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Physical properties
(i) It is a white crystalline solid. It melts at \[{{188}^{o}}C\]
(ii) It is less soluble in water. It is comparatively more soluble in alcohol.
(3) Chemical Properties : Succinic acid gives the usual reactions of dicarboxylic acid, some important reactions are:
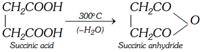
(i) Action of heat : At \[{{300}^{o}}C\]
(ii) With ammonia
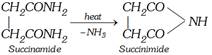
\[\underset{C{{H}_{2}}COOH}{\overset{C{{H}_{2}}COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{N{{H}_{3}}}\underset{\text{Ammonium succinate}}{\mathop{\underset{C{{H}_{2}}COON{{H}_{4}}}{\overset{C{{H}_{2}}COON{{H}_{4}}}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\text{heat}}}}\,\]
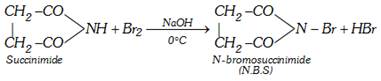
(iii) Reaction with \[B{{r}_{2}}\]
(iv) Reaction with ethylene glycol
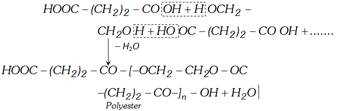
When sodium or potassium salt in aqueous solution is electrolysed, ethylene is obtained at anode.
(4) Uses : It finds use in volumetric analysis, medicine and in the manufacture of dyes, perfumes and polyester resins.
Adipic Acid or Hexane-1,6 –Dioic Acid
\[\underset{C{{H}_{2}}-C{{H}_{2}}-COOH}{\overset{C{{H}_{2}}-C{{H}_{2}}-COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\]or\[{{(C{{H}_{2}})}_{4}}{{(COOH)}_{2}}\]or\[({{C}_{6}}{{H}_{10}}{{O}_{4}})\]
It was first obtained by the oxidation of fats (Latin, adeps = fat.)
(1) Methods of Preparation
(i) From benzene (In industries)

(ii) From tetrahydrofuran (THF)
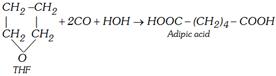
(2) Physical Properties
(i) It is a white crystalline solid. Its melting point is \[{{150}^{o}}C\].
(ii) It is fairly soluble in alcohol and ether but less soluble in water.
(3) Chemical Properties
It shows all the general reaction of dicarboxylic acids.
(i) Action of heat
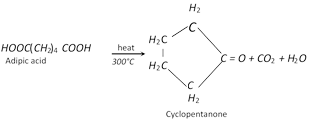
(ii) Formation of Nylon-66 [Reaction with hexa methylene diamine]
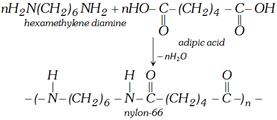
(4) Uses : It is used in the manufacture of several polymers.
Unsaturated Acids : When the double bond presents in the carbon chain of an acid is called unsaturated acid.
Example: \[\underset{\text{Acrylic acid}}{\mathop{C{{H}_{2}}=CH-COOH}}\,+\underset{\text{Maleic acid}}{\mathop{\underset{\,\,H-C-COOH}{\mathop{H-\underset{|\,|}{\mathop{C}}\,-COOH}}\,}}\,\]
Acrylic Acid or Prop-2-Enoic Acid
\[C{{H}_{2}}=CH-COOH\] or \[({{C}_{3}}{{H}_{4}}{{O}_{2}})\]
(1) Methods of Preparation
(i) From allyl alcohol
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|\,|}{\mathop{C}}}\,H\,\,\,\,\,\,\,\,}}}\,\xrightarrow{B{{r}_{2}}}\underset{\,\,\,\,C{{H}_{2}}OH}{\overset{C{{H}_{2}}Br}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HBr}}}\,\underset{[O]}{\mathop{\xrightarrow{HN{{O}_{3}}}}}\,\underset{\,\,\,COOH}{\overset{C{{H}_{2}}Br}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HBr}}}\,\underset{\text{hea}\text{t}}{\mathop{\xrightarrow{Zn}}}\,\underset{COOH}{\overset{C{{H}_{2}}\,\,\,\,\,}{\mathop{\underset{|}{\overset{|\,|}{\mathop{C}}}\,H\,\,\,\,\,\,\,}}}\,\]
(ii) By oxidation of acrolein
\[C{{H}_{2}}=CHCHO+[O]\underset{N{{H}_{4}}OH}{\mathop{\xrightarrow{AgN{{O}_{3}}}}}\,C{{H}_{2}}=CHCOOH\]
(iii) From propionic acid : \[\underset{\text{Propionic acid}}{\mathop{C{{H}_{3}}C{{H}_{2}}COOH}}\,\underset{HVZ\text{ reaction}}{\mathop{\xrightarrow{{B{{r}_{2}}}/{P}\;}}}\,\]
\[\underset{\alpha \text{-Bromopropionic acid}}{\mathop{C{{H}_{3}}CHBrCOOH}}\,\xrightarrow{Alc.KOH}C{{H}_{2}}=CHCOOH\]
(iv) By heating b-hydroxy propionic acid
\[\underset{\beta \text{-hydroxy propionic acid}}{\mathop{\underset{OH\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-C{{H}_{2}}-COOH}}\,\underset{\text{heat, }-{{H}_{2}}O}{\mathop{\xrightarrow{ZnC{{l}_{2}}}}}\,C{{H}_{2}}=CH-COOH\]
(v) From vinyl cyanide
\[\underset{\text{Acetylene}}{\mathop{HC\equiv CH}}\,+HCN\underset{90{}^\circ C}{\mathop{\xrightarrow{{C{{u}_{2}}C{{l}_{2}}}/{HCl}\;}}}\,\underset{\text{Vinyl cyanide}}{\mathop{C{{H}_{2}}=CH-CN}}\,\] [\xrightarrow{{{{H}^{+}}}/{{{H}_{2}}O}\;}C{{H}_{2}}=CH-COOH\]
(vi) From ethylene cyanohydrin
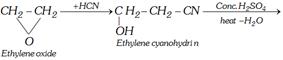
\[\underset{\begin{smallmatrix} \text{Vinyl cyanide} \\ \text{(acrylonitrile)} \end{smallmatrix}}{\mathop{C{{H}_{2}}=CH-CN}}\,\xrightarrow{{{{H}^{+}}}/{{{H}_{2}}O}\;}C{{H}_{2}}=CHCOOH\]
Industrial method : This is a new method of its manufacture.
\[CH\equiv CH+CO+{{H}_{2}}O\xrightarrow{Ni{{(CO)}_{4}}}C{{H}_{2}}=CHCOOH\]
(2) Physical Properties
(3) Chemical Properties
(i) With nascent hydrogen (Na and \[{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{OH}\])
\[C{{H}_{2}}=CHCOOH+2[H]\xrightarrow{Ni}C{{H}_{3}}C{{H}_{2}}COOH\]
(ii) With halogens and halogen acids : Markownikoff's rule is not followed.
\[C{{H}_{2}}=CHCOOH+B{{r}_{2}}\xrightarrow{CC{{l}_{4}}}\underset{\alpha \text{,}\beta \text{-Dibromopropionic acid}}{\mathop{C{{H}_{2}}Br-CHBrCOOH}}\,\]
\[C{{H}_{2}}=CHCOOH+HBr\to \underset{\beta \text{-Bromopropionic acid}}{\mathop{BrC{{H}_{2}}-C{{H}_{2}}COOH}}\,\]
(iii) Oxidation : In presence of dilute alkaline \[KMn{{O}_{4}}\].
\[C{{H}_{2}}=CHCOOH+[O]+{{H}_{2}}O\to \underset{\text{Glyceric acid}}{\mathop{C{{H}_{2}}OHCHOHCOOH}}\,\]
(iv) Salt formation
\[C{{H}_{2}}=CHCOOH+KOH\to C{{H}_{2}}=CHCO\bar{O}{{K}^{+}}+{{H}_{2}}O\]
\[2C{{H}_{2}}=CHCOOH+N{{a}_{2}}C{{O}_{3}}\to \] \[\underset{\text{Sodium acrylate}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{2C{{H}_{2}}=CHCO\bar{O}N{{a}^{+}}}}\,+{{H}_{2}}O+C{{O}_{2}}\]
(v) Ester formation
\[C{{H}_{2}}=CHCOOH+HO{{C}_{2}}{{H}_{5}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\text{Conc}\text{.}{{H}_{2}}S{{O}_{4}}}}}\,\] \[C{{H}_{2}}=\underset{\text{Ethyl acrylate}}{\mathop{CH-COO{{C}_{2}}{{H}_{5}}}}\,\]
(vi) With \[\mathbf{PC}{{\mathbf{l}}_{\mathbf{5}}}\]
\[C{{H}_{2}}=CHCOOH+PC{{l}_{5}}\to \underset{\text{Acryl chloride}}{\mathop{C{{H}_{2}}=CH-COCl}}\,\]
(4) Uses : Its ester are used for making plastics such as Lucite and plexiglass.
You need to login to perform this action.
You will be redirected in
3 sec
