Electrovalent Bond
Category : JEE Main & Advanced
An electrovalent bond is formed when a metal atom transfers one or more electrons to a non-metal atom.
\[\overset{\bullet \,\,\,\,}{\mathop{Na}}\,\,\,\,\,+\,\,\,\bullet \underset{\bullet \,\,\bullet \,\,\,}{\overset{\bullet \,\,\bullet \,\,\,\,}{\mathop{Cl\,_{\bullet }^{\bullet }}}}\,\,\,\,\,\,\,\,\,\to \,\,\,\,\,{{\left[ \underset{\,}{\overset{\,}{\mathop{Na}}}\, \right]}^{+}}\,\,{{\left[ _{\bullet }^{\bullet }\underset{\bullet \,\,\bullet \,\,}{\overset{\bullet \,\,\bullet \,\,\,}{\mathop{Cl\,_{\bullet }^{\bullet }}}}\, \right]}^{-}}\,\,\,\,\text{or}\,\,\,\,\,\,N{{a}^{+}}C{{l}^{-}}\]
Some other examples are:
\[MgC{{l}_{2}},\text{ }CaC{{l}_{2}},\text{ }MgO,\text{ }N{{a}_{2}}S,\text{ }Ca{{H}_{2}},\text{ }Al{{F}_{3}},\text{ }NaH,\text{ }KH,{{K}_{2}}O,\text{ }KI,\text{ }RbCl,\text{ }NaBr,\text{ }Ca{{H}_{2}}\] etc.
(1) Conditions for formation of electrovalent bond
(i) The atom which changes into cation (+ ive ion) should possess 1, 2 or 3 valency electrons. The other atom which changes into anion (+ ve ion) should possess 5, 6 or 7 electrons in the valency shell.
(ii) A high difference of electronegativity (about 2) of the two atoms is necessary for the formation of an electrovalent bond. Electrovalent bond is not possible between similar atoms.
(iii) There must be overall decrease in energy i.e., energy must be released. For this an atom should have low value of Ionisation potential and the other atom should have high value of electron affinity.
(iv) Higher the lattice energy, greater will be the case of forming an ionic compound. The amount of energy released when free ions combine together to form one mole of a crystal is called lattice energy (U). Lattice energy\[=\frac{K}{{{r}^{+}}+{{r}^{-}}}\]; \[{{r}^{+}}+{{r}^{-}}\] is internuclear distance.
The energy changes involved in the formation of ionic compounds from their constituent elements can be studied with the help of a thermochemical cycle called Born Haber cycle.
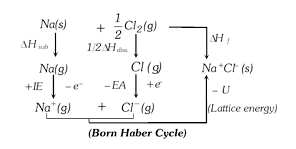
According to Hess's law of constant heat summation, heat of formation of an ionic solid is net resultant of the above changes. \[\Delta {{H}_{f}}=\Delta {{H}_{\text{Subl}\text{.}}}+\frac{1}{2}\Delta {{H}_{\text{diss}\text{.}}}+IE-EA-U\]
(2) Characteristics of electrovalent compounds
(i) Electrovalent compounds are generally crystalline is nature. The constituent ions are arranged in a regular way in their lattice.
(ii) Electrovalent compounds possess high melting and boiling points. Order of melting and boiling points in halides of sodium and oxides of IInd group elements is as,
\[NaF>NaCl>NaBr>NaI,\]\[MgO>CaO>BaO\]
(iii) Electrovalent compounds are hard and brittle in nature.
(iv) Electrovalent solids do not conduct electricity. While electrovalent compounds in the molten state or in solution conduct electricity.
(v) Electrovalent compounds are fairly soluble in polar solvents and insoluble in non-polar solvents.
(vi) The electrovalent bonds are non-rigid and non-directional. Thus these compound do not show space isomerism e.g. geometrical or optical isomerism.
(vii) Electrovalent compounds furnish ions in solution. The chemical reaction of these compounds are known as ionic reactions, which are fast.
\[{{K}^{+}}C{{l}^{-}}+\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{N{{O}_{3}}}}\,\,\,\,\xrightarrow{{}}\,\,\underset{(\text{Precipitate})}{\mathop{\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{Cl}}\,}}\,\,\downarrow +\overset{+}{\mathop{K}}\,\overset{\,-}{\mathop{N{{O}_{3}}}}\,\]
(viii) Electrovalent compounds show isomorphism.
(ix) Cooling curve of an ionic compound is not smooth, it has two break points corresponding to time of solidification.
(x) Ionic compounds show variable electrovalency due to unstability of core and inert pair effect.
You need to login to perform this action.
You will be redirected in
3 sec
