Detergents
Category : JEE Main & Advanced
As a result of high dissolving power, the naturally occurring water always contains dissolved materials, particularly ionic substances. Hard water contains certain metal ions, such as \[C{{a}^{2+}}\]and \[M{{g}^{2+}}\]. These ions react with soap. (sodium salts of stearic and similar organic acids), to produce a curdy precipitate of calcium and magnesium salts. This precipitate adheres to clothing and blocks the ability of soaps to remove oil and grease from fabrics. Synthetic detergents are very similar to the salts of fatty acids found in soap, except that they are manufactured chemically from materials others than animal fats, Examples include salts called sodium alkylbenzenesulphonates, which have the general structure.
![]()
Types of detergents
(1) Anionic detergent : Long chain alcohols are used in the manufacture of some of the synthetic anionic detergents. The long chain alcohols are treated with concentrated sulphuric acid to form alkyl hydrogen sulphates of high molecular mass and finally the alkyl sulphates are neutralized with alkali to form salts.
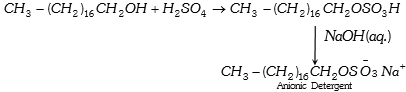
(2) Cationic detergent : These are mostly acetates or chlorides of quaternary amines. Being more expensive than the anionic detergents they find limited use. Such detergents however, possess germicidal properties and are used quite extensively as germicides. Cetyltrimethyl-ammonium chloride, is an example.
\[\left[ \underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}}\,}{\mathop{\overset{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}}\,}{\mathop{C{{H}_{3}}{{(C{{H}_{2}})}_{15}}-N-C{{H}_{3}}}}\,}}\, \right]\ C{{l}^{-}}\]
Cationic Detergent
(3) Non ionic detergent : Esters of high molecular mass formed by reactions between polyethylene glycol and stearic acid.
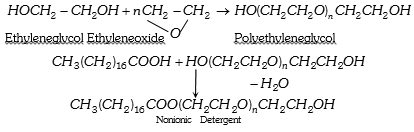
Some liquid dishwashing detergents are of nonionic type.
![]()
Detergent molecules associated with branched hydrocarbon tail which is a source of pollution.
The hydrocarbon side chain stops bacteria from attacking and breaking the chains. This results in slow degradation of detergent molecules leading to their accummulation. These days the amount of branching can be kept to a minimum. Unbranched chains are more prone to attack by bacteria so the detergents are more easily biodegraded and pollution is prevented.
You need to login to perform this action.
You will be redirected in
3 sec
